A Systematic Review on the Clinical Pharmacokinetics of Cephalexin in Healthy and Diseased Populations
- PMID: 37760698
- PMCID: PMC10526061
- DOI: 10.3390/antibiotics12091402
A Systematic Review on the Clinical Pharmacokinetics of Cephalexin in Healthy and Diseased Populations
Abstract
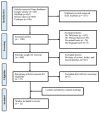
Cephalexin is a first-generation β-lactam antibiotic used in adults and pediatrics to treat various streptococcal and staphylococcal infections. This review aims to summarize and evaluate all the pharmacokinetic (PK) data on cephalexin by screening out all pertinent studies in human beings following the per oral (PO) route. By employing different online search engines such as Google Scholar, PubMed, Cochrane Central, and Science Direct, 23 studies were retrieved, among which nine were in healthy subjects, five in diseased ones, and the remaining were drug-drug, drug-food, and bioequivalence-related. These studies were included only based on the presence of plasma concentration-time profiles or PK parameters, i.e., maximum plasma concentration (Cmax), half-life (t1/2) area under the curve from time 0-infinity (AUC0-∞), and clearance (CL/F). A dose-proportional increase in AUC0-∞ and Cmax can be portrayed in different studies conducted in the healthy population. In comparison to cefaclor, Cmax was recorded to be 0.5 folds higher for cephalexin in the case of renal impairment. An increase in AUC0-∞ was seen in cephalexin on administration with probenecid, i.e., 117 µg.h/mL vs. 68.1 µg.h/mL. Moreover, drug-drug interactions with omeprazole, ranitidine, zinc sulfate, and drug-food interactions for cephalexin and other cephalosporins have also been depicted in different studies with significant changes in all PK parameters. This current review has reported all accessible studies containing PK variables in healthy and diseased populations (renal, dental, and osteoarticular infections, continuous ambulatory peritoneal dialysis) that may be favorable for health practitioners in optimizing doses among the latter.
Keywords: cephalexin; clearance; pharmacokinetics; renal disease; systematic review.
Conflict of interest statement
The authors declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Figures
References
-
- Herman T.F.H.M. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2023. [(accessed on 30 March 2023)]. Cephalexin. [Updated 2022 Aug 18] Available online: https://www.ncbi.nlm.nih.gov/books/NBK549780/
-
- PubChem Compound Summary for CID 27447, Cephalexin. [(accessed on 21 June 2023)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cephalexin.
-
- Derrick C.W., Jr., Reilly K. The role of cephalexin in the treatment of skin and soft-tissue infections. Postgrad. Med. J. 1983;59((Suppl. S5)):43–46. - PubMed
-
- Valent A.M., DeArmond C., Houston J.M., Reddy S., Masters H.R., Gold A., Boldt M., DeFranco E., Evans A.T., Warshak C.R. Effect of Post-Cesarean Delivery Oral Cephalexin and Metronidazole on Surgical Site Infection among Obese Women: A Randomized Clinical Trial. JAMA. 2017;318:1026–1034. doi: 10.1001/jama.2017.10567. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical