Chlorhexidine-Containing Electrospun Polymeric Nanofibers for Dental Applications: An In Vitro Study
- PMID: 37760711
- PMCID: PMC10526102
- DOI: 10.3390/antibiotics12091414
Chlorhexidine-Containing Electrospun Polymeric Nanofibers for Dental Applications: An In Vitro Study
Abstract
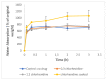
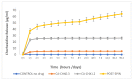
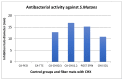
Chlorhexidine is the most commonly used anti-infective drug in dentistry. To treat infected void areas, a drug-loaded material that swells to fill the void and releases the drug slowly is needed. This study investigated the encapsulation and release of chlorhexidine from cellulose acetate nanofibers for use as an antibacterial treatment for dental bacterial infections by oral bacteria Streptococcus mutans and Enterococcus faecalis. This study used a commercial electrospinning machine to finely control the manufacture of thin, flexible, chlorhexidine-loaded cellulose acetate nanofiber mats with very-small-diameter fibers (measured using SEM). Water absorption was measured gravimetrically, drug release was analyzed by absorbance at 254 nm, and antibiotic effects were measured by halo analysis in agar. Slow electrospinning at lower voltage (14 kV), short target distance (14 cm), slow traverse and rotation, and syringe injection speeds with controlled humidity and temperature allowed for the manufacture of strong, thin films with evenly cross-meshed, uniform low-diameter nanofibers (640 nm) that were flexible and absorbed over 600% in water. Chlorhexidine was encapsulated efficiently and released in a controlled manner. All formulations killed both bacteria and may be used to fill infected voids by swelling for intimate contact with surfaces and hold the drug in the swollen matrix for effective bacterial killing in dental settings.
Keywords: anti-bacterial; chlorhexidine; controlled release preparation; nanofiber.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Electrospun Cellulose Acetate/Poly(Vinyl Alcohol) Nanofibers Loaded with Methyl Gallate and Gallic Acid for Anti-Staphylococcus aureus Applications.Polymers (Basel). 2024 Oct 23;16(21):2971. doi: 10.3390/polym16212971. Polymers (Basel). 2024. PMID: 39518181 Free PMC article.
-
Formulation, Characterization and In vitro Cytotoxicity of 5-Fluorouracil Loaded Polymeric Electrospun Nanofibers for the Treatment of Skin Cancer.Recent Pat Nanotechnol. 2019;13(2):114-128. doi: 10.2174/1872210513666190314095643. Recent Pat Nanotechnol. 2019. PMID: 30868972
-
Doxycycline release and antibacterial activity from PMMA/PEO electrospun fiber mats.J Appl Oral Sci. 2019 Oct 7;27:e20180663. doi: 10.1590/1678-7757-2018-0663. eCollection 2019. J Appl Oral Sci. 2019. PMID: 31596368 Free PMC article.
-
Fabrication of cellulose acetate/gelatin-eugenol core-shell structured nanofiber films for active packaging materials.Colloids Surf B Biointerfaces. 2022 Oct;218:112743. doi: 10.1016/j.colsurfb.2022.112743. Epub 2022 Aug 6. Colloids Surf B Biointerfaces. 2022. PMID: 35961113
-
Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances.Carbohydr Polym. 2018 Oct 15;198:131-141. doi: 10.1016/j.carbpol.2018.06.072. Epub 2018 Jun 18. Carbohydr Polym. 2018. PMID: 30092983 Review.
Cited by
-
Lysozyme-dequalinium encapsulation in a Zn-Fe LDH-chia seed matrix for enhanced antimicrobial dental therapeutics.RSC Adv. 2025 Aug 29;15(37):30872-30899. doi: 10.1039/d5ra04212g. eCollection 2025 Aug 22. RSC Adv. 2025. PMID: 40895738 Free PMC article.
-
Utilization of cellulose nanofiber in dental applications: A systematic review of in vitro evidence.Jpn Dent Sci Rev. 2025 Dec;61:103-111. doi: 10.1016/j.jdsr.2025.05.002. Epub 2025 May 31. Jpn Dent Sci Rev. 2025. PMID: 40510762 Free PMC article. Review.
-
Study of PVP and PLA Systems and Fibers Obtained by Solution Blow Spinning for Chlorhexidine Release.Polymers (Basel). 2025 Jun 30;17(13):1839. doi: 10.3390/polym17131839. Polymers (Basel). 2025. PMID: 40647849 Free PMC article.
-
Electrospun nanofibers: Focus on local therapeutic delivery targeting infectious disease.J Drug Deliv Sci Technol. 2025 Feb;104:106520. doi: 10.1016/j.jddst.2024.106520. Epub 2024 Dec 20. J Drug Deliv Sci Technol. 2025. PMID: 39802685
References
-
- Leung V., Ko F. Biomedical Applications of Nanofibers. Polym. Adv. Technol. 2011;22:350–365. doi: 10.1002/pat.1813. - DOI
-
- Petrik S. Industrial Production Technology for Nanofibers. In: Lin T., editor. Nanofibers—Production, Properties and Functional Applications. InTech; Houston, TX, USA: 2011.
LinkOut - more resources
Full Text Sources

