Procalcitonin-Based Antibiotic Use for Neonatal Early-Onset Bacterial Infections: Pre- and Post-Intervention Clinical Study
- PMID: 37760722
- PMCID: PMC10525994
- DOI: 10.3390/antibiotics12091426
Procalcitonin-Based Antibiotic Use for Neonatal Early-Onset Bacterial Infections: Pre- and Post-Intervention Clinical Study
Abstract
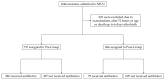
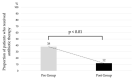
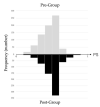
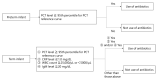
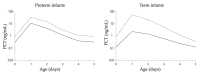
We previously reported the 95th percentile cutoff value of the serum procalcitonin (PCT) reference curve for diagnosing early-onset bacterial infection. We aimed to verify the effectivity of these novel diagnostic criteria by comparing antibiotic use and incidence of early-onset bacterial infection between pre- and post-introduction periods. We included newborns admitted to our neonatal intensive care unit who underwent blood tests within 72 h after birth between 2018 and 2022. The neonates were divided into the pre-intervention (admitted before the introduction, n = 737) or post-intervention (admitted after the introduction, n = 686) group. The days of antibiotics therapy (DOT) per 1000 patient days up to 6 days after birth, percentage of antibiotic use, and incidence of early-onset bacterial infection were compared between the groups. The post-intervention group had significantly lower DOT per 1000 patient days (82.0 days vs. 211.3 days, p < 0.01) and percentage of newborns receiving antibiotics compared with the pre-intervention group (79 (12%) vs. 280 (38%), respectively, p < 0.01). The incidence of early-onset bacterial infections did not differ between the groups (2% each, p = 0.99). In conclusion, our diagnostic criteria using the 95th percentile cutoff value of the serum PCT reference curve for early-onset bacterial infection were proven safe and effective, promoting appropriate use of antibiotics.
Keywords: antibiotic resistance; antibiotics; appropriate use of antibiotics; days of antibiotic therapy; early-onset bacterial infection; procalcitonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Detection of Bacterial Infection Based on Age-Specific Percentile-Based Reference Curve for Serum Procalcitonin Level in Preterm Infants.Clin Lab. 2020 Jan 1;66(1). doi: 10.7754/Clin.Lab.2019.190614. Clin Lab. 2020. PMID: 32013354
-
The Predictive Value of Procalcitonin and High-Sensitivity C-Reactive Protein for Early Bacterial Infections in Preterm Neonates.Neonatology. 2021;118(1):28-36. doi: 10.1159/000512523. Epub 2020 Dec 16. Neonatology. 2021. PMID: 33326974
-
Antibiotic stewardship: Early discontinuation of antibiotics based on procalcitonin level in COVID-19 pneumonia.J Clin Pharm Ther. 2022 Feb;47(2):243-247. doi: 10.1111/jcpt.13554. Epub 2021 Nov 11. J Clin Pharm Ther. 2022. PMID: 34766357
-
Potential of serum procalcitonin in predicting bacterial exacerbation and guiding antibiotic administration in severe COPD exacerbations: a systematic review and meta-analysis.Infect Dis (Lond). 2019 Sep;51(9):639-650. doi: 10.1080/23744235.2019.1644456. Epub 2019 Jul 29. Infect Dis (Lond). 2019. PMID: 31355690
-
The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills?Surg Infect (Larchmt). 2013 Dec;14(6):489-511. doi: 10.1089/sur.2012.028. Epub 2013 Nov 25. Surg Infect (Larchmt). 2013. PMID: 24274059 Review.
References
-
- Tackling Drug-Resistant Infections Globally final report and recommendations. [(accessed on 1 September 2023)]. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c....
-
- Calil R., Marba S.T.M., von Nowakonski A., Tresoldi A.T. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am. J. Infect. Control. 2001;29:133–138. doi: 10.1067/mic.2001.114223. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

