Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013-2022: Data from the European Registry on H. pylori Management (Hp-EuReg)
- PMID: 37760723
- PMCID: PMC10525558
- DOI: 10.3390/antibiotics12091427
Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013-2022: Data from the European Registry on H. pylori Management (Hp-EuReg)
Abstract
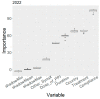
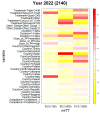
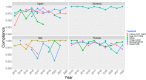
The segmentation of patients into homogeneous groups could help to improve eradication therapy effectiveness. Our aim was to determine the most important treatment strategies used in Europe, to evaluate first-line treatment effectiveness according to year and country. Data collection: All first-line empirical treatments registered at AEGREDCap in the European Registry on Helicobacter pylori management (Hp-EuReg) from June 2013 to November 2022. A Boruta method determined the "most important" variables related to treatment effectiveness. Data clustering was performed through multi-correspondence analysis of the resulting six most important variables for every year in the 2013-2022 period. Based on 35,852 patients, the average overall treatment effectiveness increased from 87% in 2013 to 93% in 2022. The lowest effectiveness (80%) was obtained in 2016 in cluster #3 encompassing Slovenia, Lithuania, Latvia, and Russia, treated with 7-day triple therapy with amoxicillin-clarithromycin (92% of cases). The highest effectiveness (95%) was achieved in 2022, mostly in Spain (81%), with the bismuth-quadruple therapy, including the single-capsule (64%) and the concomitant treatment with clarithromycin-amoxicillin-metronidazole/tinidazole (34%) with 10 (69%) and 14 (32%) days. Cluster analysis allowed for the identification of patients in homogeneous treatment groups assessing the effectiveness of different first-line treatments depending on therapy scheme, adherence, country, and prescription year.
Keywords: Helicobacter pylori; clustering; eradication; machine learning; phenotyping; treatment.
Conflict of interest statement
Javier P. Gisbert has served as a speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen. Olga P. Nyssen has received research funding from Mayoly and Allergan. The remaining authors have declared no conflicts of interest.
Figures


Similar articles
-
Empirical Second-Line Therapy in 5000 Patients of the European Registry on Helicobacter pylori Management (Hp-EuReg).Clin Gastroenterol Hepatol. 2022 Oct;20(10):2243-2257. doi: 10.1016/j.cgh.2021.12.025. Epub 2021 Dec 23. Clin Gastroenterol Hepatol. 2022. PMID: 34954341
-
Helicobacter pylori Eradication Rates in Slovenia in the Period from 2017 to 2019: Data from the European Registry on H. pylori Management.Dig Dis. 2021;39(4):318-324. doi: 10.1159/000512506. Epub 2020 Oct 23. Dig Dis. 2021. PMID: 33099549
-
[Effectiveness of empirical Helicobacter pylori eradication therapy with furazolidone in Russia: results from the European Registry on Helicobacter pylori Management (Hp-EuReg)].Ter Arkh. 2023 Mar 30;95(2):120-129. doi: 10.26442/00403660.2023.02.202107. Ter Arkh. 2023. PMID: 37167127 Russian.
-
Helicobacter pylori-related diseases.Gastroenterol Hepatol. 2016 Sep;39 Suppl 1:36-46. doi: 10.1016/S0210-5705(16)30173-X. Gastroenterol Hepatol. 2016. PMID: 27888863 Review. English, Spanish.
-
'Rescue' therapies for the management of Helicobacter pylori infection.Dig Dis. 2006;24(1-2):113-30. doi: 10.1159/000090315. Dig Dis. 2006. PMID: 16699270 Review.
Cited by
-
The Helicobacter pylori AI-clinician harnesses artificial intelligence to personalise H. pylori treatment recommendations.Nat Commun. 2025 Jul 14;16(1):6472. doi: 10.1038/s41467-025-61329-5. Nat Commun. 2025. PMID: 40659612 Free PMC article.
-
Cloning, Expression, Purification and Biological Activity Analysis of Recombinant Helicobacter pylori FabI as a Drug Target.Mol Biotechnol. 2025 Feb 27. doi: 10.1007/s12033-025-01411-x. Online ahead of print. Mol Biotechnol. 2025. PMID: 40016568
-
Prevalence of Helicobacter pylori Infection and Micronutrient Deficiencies in a Clinically Referred Cohort of Ezidi Refugees in Rural Armidale: Findings from a Retrospective Study.J Immigr Minor Health. 2025 Jul 1. doi: 10.1007/s10903-025-01715-9. Online ahead of print. J Immigr Minor Health. 2025. PMID: 40591143
-
Best Practices for Helicobacter pylori Management.Gastroenterol Hepatol (N Y). 2024 Mar;20(3):159-168. Gastroenterol Hepatol (N Y). 2024. PMID: 38680170 Free PMC article.
-
Machine Learning-Based Prediction of Helicobacter pylori Infection Study in Adults.Med Sci Monit. 2024 Jun 8;30:e943666. doi: 10.12659/MSM.943666. Med Sci Monit. 2024. PMID: 38850016 Free PMC article.
References
-
- Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. - DOI - PubMed
-
- McColl K.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2010;362:1597–1604. - PubMed
-
- Mégraud F., Graham D.Y., Howden C.W., Trevino E., Weissfeld A., Hunt B., Smith N., Leifke E., Chey W.D. Rates of Antimicrobial Resistance in Helicobacter pylori Isolates from Clinical Trial Patients Across the US and Europe. Am. J. Gastroenterol. 2023;118:269–275. doi: 10.14309/ajg.0000000000002045. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

