Detection, Identification and Diagnostic Characterization of the Staphylococcal Small Colony-Variant (SCV) Phenotype
- PMID: 37760742
- PMCID: PMC10525764
- DOI: 10.3390/antibiotics12091446
Detection, Identification and Diagnostic Characterization of the Staphylococcal Small Colony-Variant (SCV) Phenotype
Abstract
While modern molecular methods have decisively accelerated and improved microbiological diagnostics, phenotypic variants still pose a challenge for their detection, identification and characterization. This particularly applies if they are unstable and hard to detect, which is the case for the small-colony-variant (SCV) phenotype formed by staphylococci. On solid agar media, staphylococcal SCVs are characterized by tiny colonies with deviant colony morphology. Their reduced growth rate and fundamental metabolic changes are the result of their adaptation to an intracellular lifestyle, regularly leading to specific auxotrophies, such as for menadione, hemin or thymidine. These alterations make SCVs difficult to recognize and render physiological, biochemical and other growth-based methods such as antimicrobial susceptibility testing unreliable or unusable. Therefore, diagnostic procedures require prolonged incubation times and, if possible, confirmation by molecular methods. A special approach is needed for auxotrophy testing. However, standardized protocols for SCV diagnostics are missing. If available, SCVs and their putative parental isolates should be genotyped to determine clonality. Since their detection has significant implications for the treatment of the infection, which is usually chronic and relapsing, SCV findings should be specifically reported, commented on, and managed in close collaboration with the microbiological laboratory and the involved clinicians.
Keywords: Staphylococcus aureus; auxotrophy; chronic infection; coagulase-negative staphylococci; cultivation; diagnostics; identification; intracellular; relapse; small-colony-variant.
Conflict of interest statement
The author declares no conflict of interest.
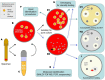
Figures


Similar articles
-
Staphylococcus aureus small colony variants show common metabolic features in central metabolism irrespective of the underlying auxotrophism.Front Cell Infect Microbiol. 2014 Oct 21;4:141. doi: 10.3389/fcimb.2014.00141. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25374845 Free PMC article.
-
Optimized In Vitro Antibiotic Susceptibility Testing Method for Small-Colony Variant Staphylococcus aureus.Antimicrob Agents Chemother. 2016 Jan 4;60(3):1725-35. doi: 10.1128/AAC.02330-15. Antimicrob Agents Chemother. 2016. PMID: 26729501 Free PMC article.
-
Characterization of Staphylococcus epidermidis and Staphyloccocus warneri small-colony variants associated with prosthetic-joint infections.J Med Microbiol. 2014 Feb;63(Pt 2):176-185. doi: 10.1099/jmm.0.066068-0. Epub 2013 Nov 20. J Med Microbiol. 2014. PMID: 24257683
-
Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians.Int J Antimicrob Agents. 2008 Jun;31(6):507-10. doi: 10.1016/j.ijantimicag.2007.10.026. Epub 2008 Jan 3. Int J Antimicrob Agents. 2008. PMID: 18180148 Review.
-
Small colony variants of Staphylococcus aureus--review.Folia Microbiol (Praha). 2010 Nov;55(6):548-58. doi: 10.1007/s12223-010-0089-3. Epub 2011 Jan 21. Folia Microbiol (Praha). 2010. PMID: 21253898 Review.
Cited by
-
Potential in-host evolution of Klebsiella pneumoniae ST147: convergence and the role of capsular alterations in morphotype diversity.Microbiol Spectr. 2025 Sep 2;13(9):e0017025. doi: 10.1128/spectrum.00170-25. Epub 2025 Jul 18. Microbiol Spectr. 2025. PMID: 40679293 Free PMC article.
-
The Controversial Effect of Antibiotics on Methicillin-Sensitive S. aureus: A Comparative In Vitro Study.Int J Mol Sci. 2023 Nov 14;24(22):16308. doi: 10.3390/ijms242216308. Int J Mol Sci. 2023. PMID: 38003500 Free PMC article.
-
Integrative omics analysis reveals insights into small colony variants of Staphylococcus aureus induced by sulfamethoxazole-trimethoprim.BMC Microbiol. 2024 Jun 14;24(1):212. doi: 10.1186/s12866-024-03364-8. BMC Microbiol. 2024. PMID: 38877418 Free PMC article.
-
Nutritional Stress Leads to Persistence and Persister-like Growth in Staphylococcus aureus.Pathogens. 2025 Mar 4;14(3):251. doi: 10.3390/pathogens14030251. Pathogens. 2025. PMID: 40137735 Free PMC article.
-
Exploring the antibacterial and anti-biofilm properties of Diacerein against methicillin-resistant Staphylococcus aureus.Front Microbiol. 2025 Mar 20;16:1545902. doi: 10.3389/fmicb.2025.1545902. eCollection 2025. Front Microbiol. 2025. PMID: 40182283 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

