Effect of Hydrogen Peroxide on Cyanobacterial Biofilms
- PMID: 37760746
- PMCID: PMC10525773
- DOI: 10.3390/antibiotics12091450
Effect of Hydrogen Peroxide on Cyanobacterial Biofilms
Abstract
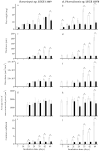
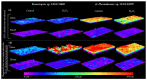
Although a range of disinfecting formulations is commercially available, hydrogen peroxide is one of the safest chemical agents used for disinfection in aquatic environments. However, its effect on cyanobacterial biofilms is poorly investigated. In this work, biofilm formation by two filamentous cyanobacterial strains was evaluated over seven weeks on two surfaces commonly used in marine environments: glass and silicone-based paint (Sil-Ref) under controlled hydrodynamic conditions. After seven weeks, the biofilms were treated with a solution of hydrogen peroxide (H2O2) to assess if disinfection could affect long-term biofilm development. The cyanobacterial biofilms appeared to be tolerant to H2O2 treatment, and two weeks after treatment, the biofilms that developed on glass by one of the strains presented higher biomass amounts than the untreated biofilms. This result emphasizes the need to correctly evaluate the efficiency of disinfection in cyanobacterial biofilms, including assessing the possible consequences of inefficient disinfection on the regrowth of these biofilms.
Keywords: antifouling strategies; chemical disinfection; cyanobacterial biofilms; hydrogen peroxide; marine biofouling; resistance; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Unveiling the Antifouling Performance of Different Marine Surfaces and Their Effect on the Development and Structure of Cyanobacterial Biofilms.Microorganisms. 2021 May 20;9(5):1102. doi: 10.3390/microorganisms9051102. Microorganisms. 2021. PMID: 34065462 Free PMC article.
-
Characterization and biofouling potential analysis of two cyanobacterial strains isolated from Cape Verde and Morocco.FEMS Microbiol Ecol. 2023 Feb 28;99(3):fiad004. doi: 10.1093/femsec/fiad004. FEMS Microbiol Ecol. 2023. PMID: 36633537
-
Quantitative proteomic analysis of marine biofilms formed by filamentous cyanobacterium.Environ Res. 2021 Oct;201:111566. doi: 10.1016/j.envres.2021.111566. Epub 2021 Jun 25. Environ Res. 2021. PMID: 34181917
-
Biofouling and me: My Stockholm syndrome with biofilms.Water Res. 2020 Apr 15;173:115576. doi: 10.1016/j.watres.2020.115576. Epub 2020 Feb 2. Water Res. 2020. PMID: 32044598 Review.
-
Prevention of marine biofouling using natural compounds from marine organisms.Biotechnol Annu Rev. 2000;6:221-41. doi: 10.1016/s1387-2656(00)06024-5. Biotechnol Annu Rev. 2000. PMID: 11193296 Review.
References
Grants and funding
- LA/P/0045/2020/ALiCE
- UIDB/00511/2020 and UIDP/00511/2020/LEPABE
- project HealthyWaters (NORTE-01-0145-FEDER-000069)/Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF);
- Project SurfSAFE under grant agreement no. 952471/European Union's Horizon 2020 Research and Innovation Programme
- Project BacAllFree (2022.05314.PTDC)/National funds through FCT/MCTES (PIDDAC)
LinkOut - more resources
Full Text Sources

