Long Non-Coding RNAs in Colorectal Cancer: Navigating the Intersections of Immunity, Intercellular Communication, and Therapeutic Potential
- PMID: 37760852
- PMCID: PMC10525929
- DOI: 10.3390/biomedicines11092411
Long Non-Coding RNAs in Colorectal Cancer: Navigating the Intersections of Immunity, Intercellular Communication, and Therapeutic Potential
Abstract
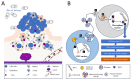
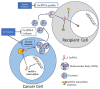
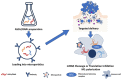
This comprehensive review elucidates the intricate roles of long non-coding RNAs (lncRNAs) within the colorectal cancer (CRC) microenvironment, intersecting the domains of immunity, intercellular communication, and therapeutic potential. lncRNAs, which are significantly involved in the pathogenesis of CRC, immune evasion, and the treatment response to CRC, have crucial implications in inflammation and serve as promising candidates for novel therapeutic strategies and biomarkers. This review scrutinizes the interaction of lncRNAs with the Consensus Molecular Subtypes (CMSs) of CRC, their complex interplay with the tumor stroma affecting immunity and inflammation, and their conveyance via extracellular vesicles, particularly exosomes. Furthermore, we delve into the intricate relationship between lncRNAs and other non-coding RNAs, including microRNAs and circular RNAs, in mediating cell-to-cell communication within the CRC microenvironment. Lastly, we propose potential strategies to manipulate lncRNAs to enhance anti-tumor immunity, thereby underlining the significance of lncRNAs in devising innovative therapeutic interventions in CRC.
Keywords: colorectal cancers (CRCs); immune cells; long non-coding RNAs (lncRNAs); tumor environment (TME).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Exosomal long non-coding RNAs in cancer: Interplay, modulation, and therapeutic avenues.Noncoding RNA Res. 2024 Apr 4;9(3):887-900. doi: 10.1016/j.ncrna.2024.03.014. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38616862 Free PMC article. Review.
-
Cross-Talk Between m6A- and m5C-Related lncRNAs to Construct a Novel Signature and Predict the Immune Landscape of Colorectal Cancer Patients.Front Immunol. 2022 Mar 8;13:740960. doi: 10.3389/fimmu.2022.740960. eCollection 2022. Front Immunol. 2022. PMID: 35350786 Free PMC article.
-
Exosomal Long Non-coding RNAs: Emerging Players in the Tumor Microenvironment.Mol Ther Nucleic Acids. 2020 Oct 4;23:1371-1383. doi: 10.1016/j.omtn.2020.09.039. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33738133 Free PMC article. Review.
-
Insights on the Biomarker Potential of Exosomal Non-Coding RNAs in Colorectal Cancer: An In Silico Characterization of Related Exosomal lncRNA/circRNA-miRNA-Target Axis.Cells. 2023 Apr 4;12(7):1081. doi: 10.3390/cells12071081. Cells. 2023. PMID: 37048155 Free PMC article. Review.
-
Role of exosomal long non-coding RNAs in colorectal cancer.World J Gastrointest Oncol. 2021 Aug 15;13(8):867-878. doi: 10.4251/wjgo.v13.i8.867. World J Gastrointest Oncol. 2021. PMID: 34457192 Free PMC article. Review.
Cited by
-
Exploring the interplay of natural products and long non-coding RNAs in colorectal cancer: pathogenesis, diagnosis, and overcoming drug resistance.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1243-1263. doi: 10.1007/s00210-024-03425-9. Epub 2024 Sep 17. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39287672 Review.
-
The roles of long non-coding RNAs in Alzheimer's disease diagnosis, treatment, and their involvement in Alzheimer's disease immune responses.Noncoding RNA Res. 2024 Mar 16;9(3):659-666. doi: 10.1016/j.ncrna.2024.03.008. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38577023 Free PMC article. Review.
-
Long non-coding RNAs (lncRNAs) in cancer development: new insight from STAT3 signaling pathway to immune evasion.Clin Exp Med. 2025 Feb 11;25(1):53. doi: 10.1007/s10238-024-01532-8. Clin Exp Med. 2025. PMID: 39932585 Free PMC article. Review.
-
Gene and lncRNA Profiling of ω3/ω6 Polyunsaturated Fatty Acid-Exposed Human Visceral Adipocytes Uncovers Different Responses in Healthy Lean, Obese and Colorectal Cancer-Affected Individuals.Int J Mol Sci. 2024 Mar 15;25(6):3357. doi: 10.3390/ijms25063357. Int J Mol Sci. 2024. PMID: 38542331 Free PMC article.
-
Immune checkpoints and ncRNAs: pioneering immunotherapy approaches for hematological malignancies.Cancer Cell Int. 2024 Dec 19;24(1):410. doi: 10.1186/s12935-024-03596-8. Cancer Cell Int. 2024. PMID: 39702293 Free PMC article. Review.
References
-
- Patel S.G., Karlitz J.J., Yen T., Lieu C.H., Boland C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022;7:262–274. doi: 10.1016/S2468-1253(21)00426-X. - DOI - PubMed
-
- Maaser C., Sturm A., Vavricka S.R., Kucharzik T., Fiorino G., Annese V., Calabrese E., Baumgart D.C., Bettenworth D., Borralho Nunes P., et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. - DOI - PubMed
-
- Kobayashi H., Gieniec K.A., Lannagan T.R.M., Wang T., Asai N., Mizutani Y., Iida T., Ando R., Thomas E.M., Sakai A., et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology. 2022;162:890–906. doi: 10.1053/j.gastro.2021.11.037. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

