Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders
- PMID: 37760865
- PMCID: PMC10525371
- DOI: 10.3390/biomedicines11092424
Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders
Abstract
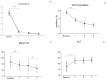
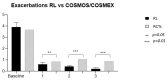
The efficacy mepolizumab in severe asthmatic patients is proven in the literature. Primarily to study the effect of mepolizumab on exacerbations, steroid dependence, and the continuation of efficacy in the long term. Secondarily to evaluate the effect of the drug on nasal polyps. Analyzing data from SANI (Severe Asthma Network Italy) clinics, we observed severe asthmatic patients treated with mepolizumab 100 mg/4 weeks, for a period of 3 years. 157 patients were observed. Exacerbations were reduced from the first year (-84.6%) and progressively to 90 and 95% in the second and third ones. Steroid-dependent patients decreased from 54% to 21% and subsequently to 11% in the second year and 6% in the third year. Patients with concomitant nasal polyps, assessed by SNOT-22, showed a 49% reduction in value from baseline to the third year. The study demonstrated the long-term efficacy of mepolizumab in a real-life setting.
Keywords: CRSwNP; IL-5; eosinophils; mepolizumab; real life; registry; severe asthma.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures

References
-
- Bonato M., Bazzan E., Snijders D., Turato G., Turrin M., Cosio M.G., Barbato A., Saetta M., Baraldo S. Innate Lymphocytes -ILC2—Might Be the Drivers of T2-High Nonatopic Asthma in Children. Eur. Respir. J. 2021;58:PA861. doi: 10.1183/13993003.CONGRESS-2021.PA861. - DOI

