Creation of an iPSC-Based Skeletal Muscle Model of McArdle Disease Harbouring the Mutation c.2392T>C (p.Trp798Arg) in the PYGM Gene
- PMID: 37760875
- PMCID: PMC10525199
- DOI: 10.3390/biomedicines11092434
Creation of an iPSC-Based Skeletal Muscle Model of McArdle Disease Harbouring the Mutation c.2392T>C (p.Trp798Arg) in the PYGM Gene
Abstract
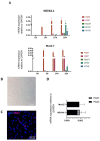
McArdle disease is a rare autosomal recessive condition caused by mutations in the PYGM gene. This gene encodes the skeletal muscle isoform of glycogen phosphorylase or myophosphorylase. Patients with McArdle disease have an inability to obtain energy from their muscle glycogen stores, which manifests as a marked exercise intolerance. Nowadays, there is no cure for this disorder and recommendations are intended to prevent and mitigate symptoms. There is great heterogeneity among the pathogenic variants found in the PYGM gene, and there is no obvious correlation between genotypes and phenotypes. Here, we present the generation of the first human iPSC-based skeletal muscle model harbouring the second most frequent mutation in PYGM in the Spanish population: NM_005609.4: c.2392T>C (p.Trp798Arg). To this end, iPSCs derived from a McArdle patient and a healthy control were both successfully differentiated into skeletal muscle cells using a small molecule-based protocol. The created McArdle skeletal muscle model was validated by confirming distinctive biochemical aspects of the disease such as the absence of myophosphorylase, the most typical biochemical feature of these patients. This model will be very valuable for use in future high-throughput pharmacological screenings.
Keywords: McArdle disease; PYGM; disease modelling; iPSCs; induced pluripotent stem cell; skeletal muscle differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Generation of the First Human In Vitro Model for McArdle Disease Based on iPSC Technology.Int J Mol Sci. 2022 Nov 12;23(22):13964. doi: 10.3390/ijms232213964. Int J Mol Sci. 2022. PMID: 36430443 Free PMC article.
-
PYGM expression analysis in white blood cells: a complementary tool for diagnosing McArdle disease?Neuromuscul Disord. 2014 Dec;24(12):1079-86. doi: 10.1016/j.nmd.2014.08.002. Epub 2014 Aug 21. Neuromuscul Disord. 2014. PMID: 25240406
-
Systemic AAV8-mediated delivery of a functional copy of muscle glycogen phosphorylase (Pygm) ameliorates disease in a murine model of McArdle disease.Hum Mol Genet. 2020 Jan 1;29(1):20-30. doi: 10.1093/hmg/ddz214. Hum Mol Genet. 2020. PMID: 31511858
-
McArdle Disease: Update of Reported Mutations and Polymorphisms in the PYGM Gene.Hum Mutat. 2015 Jul;36(7):669-78. doi: 10.1002/humu.22806. Epub 2015 Jun 3. Hum Mutat. 2015. PMID: 25914343 Review.
-
Genes and exercise intolerance: insights from McArdle disease.Physiol Genomics. 2016 Feb;48(2):93-100. doi: 10.1152/physiolgenomics.00076.2015. Epub 2015 Oct 13. Physiol Genomics. 2016. PMID: 26465709 Review.
Cited by
-
Human-Induced Pluripotent Stem Cells (iPSCs) for Disease Modeling and Insulin Target Cell Regeneration in the Treatment of Insulin Resistance: A Review.Cells. 2025 Aug 1;14(15):1188. doi: 10.3390/cells14151188. Cells. 2025. PMID: 40801620 Free PMC article. Review.
References
-
- Santalla A., Nogales-Gadea G., Encinar A.B., Vieitez I., González-Quintana A., Serrano-Lorenzo P., Consuegra I.G., Asensio S., Ballester-Lopez A., Pintos-Morell G., et al. Genotypic and phenotypic features of all Spanish patients with McArdle disease: A 2016 update. BMC Genom. 2017;18:819. doi: 10.1186/s12864-017-4188-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

