Identifying Aging-Related Biomarkers and Immune Infiltration Features in Diabetic Nephropathy Using Integrative Bioinformatics Approaches and Machine-Learning Strategies
- PMID: 37760894
- PMCID: PMC10525809
- DOI: 10.3390/biomedicines11092454
Identifying Aging-Related Biomarkers and Immune Infiltration Features in Diabetic Nephropathy Using Integrative Bioinformatics Approaches and Machine-Learning Strategies
Abstract
Background: Aging plays an essential role in the development of diabetic nephropathy (DN). This study aimed to identify and verify potential aging-related genes associated with DN using bioinformatics analysis.
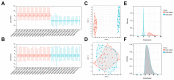
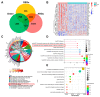
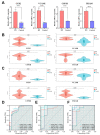
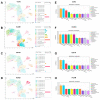
Methods: To begin with, we combined the datasets from GEO microarrays (GSE104954 and GSE30528) to find the genes that were differentially expressed (DEGs) across samples from DN and healthy patient populations. By overlapping DEGs, weighted co-expression network analysis (WGCNA), and 1357 aging-related genes (ARGs), differentially expressed ARGs (DEARGs) were discovered. We next performed functional analysis to determine DEARGs' possible roles. Moreover, protein-protein interactions were examined using STRING. The hub DEARGs were identified using the CytoHubba, MCODE, and LASSO algorithms. We next used two validation datasets and Receiver Operating Characteristic (ROC) curves to determine the diagnostic significance of the hub DEARGs. RT-qPCR, meanwhile, was used to confirm the hub DEARGs' expression levels in vitro. In addition, we investigated the relationships between immune cells and hub DEARGs. Next, Gene Set Enrichment Analysis (GSEA) was used to identify each biomarker's biological role. The hub DEARGs' subcellular location and cell subpopulations were both identified and predicted using the HPA and COMPARTMENTS databases, respectively. Finally, drug-protein interactions were predicted and validated using STITCH and AutoDock Vina.
Results: A total of 57 DEARGs were identified, and functional analysis reveals that they play a major role in inflammatory processes and immunomodulation in DN. In particular, aging and the AGE-RAGE signaling pathway in diabetic complications are significantly enriched. Four hub DEARGs (CCR2, VCAM1, CSF1R, and ITGAM) were further screened using the interaction network, CytoHubba, MCODE, and LASSO algorithms. The results above were further supported by validation sets, ROC curves, and RT-qPCR. According to an evaluation of immune infiltration, DN had significantly more resting mast cells and delta gamma T cells but fewer regulatory T cells and active mast cells. Four DEARGs have statistical correlations with them as well. Further investigation revealed that four DEARGs were implicated in immune cell abnormalities and regulated a wide range of immunological and inflammatory responses. Furthermore, the drug-protein interactions included four possible therapeutic medicines that target four DEARGs, and molecular docking could make this association practical.
Conclusions: This study identified four DEARGs (CCR2, VCAM1, CSF1R, and ITGAM) associated with DN, which might play a key role in the development of DN and could be potential biomarkers in DN.
Keywords: aging; diabetic nephropathy; diagnostic biomarker; immune cell infiltration; molecular docking.
Conflict of interest statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

