The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes
- PMID: 37760919
- PMCID: PMC10525388
- DOI: 10.3390/biomedicines11092475
The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes
Abstract
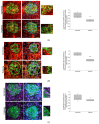
Epigenetic alterations contribute to the pathogenesis of chronic diseases such as diabetes mellitus. Previous studies of our group showed that diabetic conditions reduce the trimethylation of H3K27 in podocytes in a NIPP1- (nuclear inhibitor of protein phosphatase 1) and EZH2- (enhancer of zeste homolog 2) dependent manner. It has been previously reported that in differentiated podocytes, hypoxia decreases the expression of slit diaphragm proteins and promotes foot process effacement, thereby contributing to the progression of renal disease. The exact mechanisms are, however, not completely understood. The aim of this study was to analyze the role of hypoxia and HIFs (hypoxia-inducible factor) on epigenetic changes in podocytes affecting NIPP1, EZH2 and H3K27me3, in vitro and in vivo. In vivo studies were performed with mice exposed to 10% systemic hypoxia for 3 days or injected with 3,4-DHB (dihydroxybenzoate), a PHD (prolyl hydroxylase) inhibitor, 24 h prior analyses. Immunodetection of H3K27me3, NIPP1 and EZH2 in glomerular podocytes revealed, to the best of our knowledge for the first time, that hypoxic conditions and pharmacological HIFs activation significantly reduce the expression of NIPP1 and EZH2 and diminish H3K27 trimethylation. These findings are also supported by in vitro studies using murine-differentiated podocytes.
Keywords: EZH2; H3K27me3; NIPP1; enhancer of zeste homolog 2; epigenetic; hypoxia; nuclear inhibitor of protein phosphatase 1; podocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
AGE-Induced Suppression of EZH2 Mediates Injury of Podocytes by Reducing H3K27me3.Am J Nephrol. 2020;51(9):676-692. doi: 10.1159/000510140. Epub 2020 Aug 27. Am J Nephrol. 2020. PMID: 32854097
-
The transcriptional repressor NIPP1 is an essential player in EZH2-mediated gene silencing.Oncogene. 2008 Feb 28;27(10):1449-60. doi: 10.1038/sj.onc.1210774. Epub 2007 Sep 3. Oncogene. 2008. PMID: 17724462
-
The Histone Methyltransferase Enzyme Enhancer of Zeste Homolog 2 Protects against Podocyte Oxidative Stress and Renal Injury in Diabetes.J Am Soc Nephrol. 2016 Jul;27(7):2021-34. doi: 10.1681/ASN.2014090898. Epub 2015 Nov 3. J Am Soc Nephrol. 2016. PMID: 26534922 Free PMC article.
-
Detrimental effects of hypoxia on glomerular podocytes.J Physiol Biochem. 2021 May;77(2):193-203. doi: 10.1007/s13105-021-00788-y. Epub 2021 Apr 9. J Physiol Biochem. 2021. PMID: 33835424 Review.
-
Epigenetic Alterations in Podocytes in Diabetic Nephropathy.Front Pharmacol. 2021 Sep 24;12:759299. doi: 10.3389/fphar.2021.759299. eCollection 2021. Front Pharmacol. 2021. PMID: 34630127 Free PMC article. Review.
Cited by
-
Role of Epigenetic Changes in the Pathophysiology of Diabetic Kidney Disease.Glomerular Dis. 2024 Nov 13;4(1):211-226. doi: 10.1159/000541923. eCollection 2024 Jan-Dec. Glomerular Dis. 2024. PMID: 39649441 Free PMC article. Review.
References
-
- Wharram B.L., Goyal M., Wiggins J.E., Sanden S.K., Hussain S., Filipiak W.E., Saunders T.L., Dysko R.C., Kohno K., Holzman L.B., et al. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. - DOI - PubMed
LinkOut - more resources
Full Text Sources

