Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma-Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation
- PMID: 37760923
- PMCID: PMC10526098
- DOI: 10.3390/biomedicines11092482
Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma-Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation
Abstract
Background: In the treatment of advanced urothelial (aUC) and renal cell carcinoma (aRCC), biomarkers such as PD-1 and PD-L1 are not robust prognostic markers for immunotherapy (IO) response. Previously, a significant association between IO and a change in splenic volume (SV) was described for several tumour entities. To the best of our knowledge, this study presents the first correlation of SV to IO in aUC and aRCC.
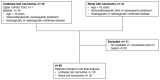
Methods: All patients with aUC (05/2017-10/2021) and aRCC (01/2012-05/2022) treated with IO at our academic centre were included. SV was measured at baseline, 3 and 9 months after initiation of IO using an in-house developed convolutional neural network-based spleen segmentation method. Uni- and multivariate Cox regression models for overall survival (OS) and progression-free survival (PFS) were used.
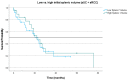
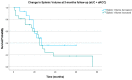
Results: In total, 35 patients with aUC and 30 patients with aRCC were included in the analysis. Lower SV at the three-month follow-up was significantly associated with improved OS in the aRCC group.
Conclusions: We describe a new, innovative artificial intelligence-based approach of a radiological surrogate marker for IO response in aUC and aRCC which presents a promising new predictive imaging marker. The data presented implicate improved OS with lower follow-up SV in patients with aRCC.
Keywords: advanced renal cell carcinoma; advanced urothelial carcinoma; artificial intelligence; immune checkpoint inhibitor; immunotherapy; predictive marker; prognostic marker; splenic volume.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Powles T., Bellmunt J., Comperat E., De Santis M., Huddart R., Loriot Y., Necchi A., Valderrama B.P., Ravaud A., Shariat S.F., et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. - DOI - PubMed
-
- Rebuzzi S.E., Banna G.L., Murianni V., Damassi A., Giunta E.F., Fraggetta F., De Giorgi U., Cathomas R., Rescigno P., Brunelli M., et al. Prognostic and Predictive Factors in Advanced Urothelial Carcinoma Treated with Immune Checkpoint Inhibitors: A Review of the Current Evidence. Cancers. 2021;13:5517. doi: 10.3390/cancers13215517. - DOI - PMC - PubMed
-
- Mori K., Pradere B., Moschini M., Mostafaei H., Laukhtina E., Schuettfort V.M., Sari Motlagh R., Soria F., Teoh J.Y.C., Egawa S., et al. First-line immune-checkpoint inhibitor combination therapy for chemotherapy-eligible patients with metastatic urothelial carcinoma: A systematic review and meta-analysis. Eur. J. Cancer. 2021;151:35–48. doi: 10.1016/j.ejca.2021.03.049. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

