UBL3 Interaction with α-Synuclein Is Downregulated by Silencing MGST3
- PMID: 37760932
- PMCID: PMC10648775
- DOI: 10.3390/biomedicines11092491
UBL3 Interaction with α-Synuclein Is Downregulated by Silencing MGST3
Abstract
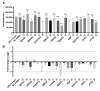
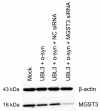
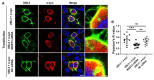
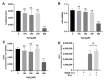
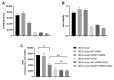
Ubiquitin-like 3 (UBL3) is a membrane-anchored protein that plays a crucial role in sorting proteins into small extracellular vesicles. Aggregations of alpha-synuclein (α-syn) are associated with the pathology of neurodegenerative diseases such as Parkinson's disease. Recently, the interaction between UBL3 and α-syn was discovered, with potential implications in clearing excess α-syn from neurons and its role in disease spread. However, the regulator that can mediate the interaction between UBL3 and α-syn remains unclear. In this study, using the split gaussian luciferase complementation assay and RNA interference technology, we identified that QSOX2, HTATIP2, UBE3C, MGST3, NSF, HECTD1, SAE1, and ATG3 were involved in downregulating the interaction between UBL3 and α-syn. Notably, silencing MGST3 had the most significant impact. Immunocytochemistry staining confirmed the impact of MGST3 silencing on the co-localization of UBL3 and α-syn in cells. MGST3 is a part of the antioxidant system, and silencing MGST3 is believed to contribute to oxidative stress. We induced oxidative stress with hydrogen peroxide, observing its effect on the UBL3-α-syn interaction, and showing that 800 µM of H2O2 downregulated this interaction. In conclusion, silencing MGST3 downregulates the interaction between UBL3 and α-syn.
Keywords: hydrogen peroxide; microsomal glutathione s-transferase 3; oxidative stress; ubiquitin-like 3; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
UBL3 Interacts with PolyQ-Expanded Huntingtin Fragments and Modifies Their Intracellular Sorting.Neurol Int. 2024 Oct 22;16(6):1175-1188. doi: 10.3390/neurolint16060089. Neurol Int. 2024. PMID: 39449505 Free PMC article.
-
Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress.Int J Mol Sci. 2024 Jul 4;25(13):7353. doi: 10.3390/ijms25137353. Int J Mol Sci. 2024. PMID: 39000460 Free PMC article.
-
UBL3 Interacts with Alpha-Synuclein in Cells and the Interaction Is Downregulated by the EGFR Pathway Inhibitor Osimertinib.Biomedicines. 2023 Jun 10;11(6):1685. doi: 10.3390/biomedicines11061685. Biomedicines. 2023. PMID: 37371780 Free PMC article.
-
Ubiquitin-like 3 as a new protein-sorting factor for small extracellular vesicles.Cell Struct Funct. 2022;47(1):1-18. doi: 10.1247/csf.21078. Cell Struct Funct. 2022. PMID: 35197392 Free PMC article. Review.
-
A New Potential Therapeutic Target for Cancer in Ubiquitin-Like Proteins-UBL3.Int J Mol Sci. 2023 Jan 8;24(2):1231. doi: 10.3390/ijms24021231. Int J Mol Sci. 2023. PMID: 36674743 Free PMC article. Review.
Cited by
-
UBL3 Interacts with PolyQ-Expanded Huntingtin Fragments and Modifies Their Intracellular Sorting.Neurol Int. 2024 Oct 22;16(6):1175-1188. doi: 10.3390/neurolint16060089. Neurol Int. 2024. PMID: 39449505 Free PMC article.
-
Alpha-Synuclein Interaction with UBL3 Is Upregulated by Microsomal Glutathione S-Transferase 3, Leading to Increased Extracellular Transport of the Alpha-Synuclein under Oxidative Stress.Int J Mol Sci. 2024 Jul 4;25(13):7353. doi: 10.3390/ijms25137353. Int J Mol Sci. 2024. PMID: 39000460 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

