The Safety Profiles of Two First-Generation NTRK Inhibitors: Analysis of Individual Case Safety Reports from the FDA Adverse Event Reporting System (FAERS) Database
- PMID: 37760979
- PMCID: PMC10526334
- DOI: 10.3390/biomedicines11092538
The Safety Profiles of Two First-Generation NTRK Inhibitors: Analysis of Individual Case Safety Reports from the FDA Adverse Event Reporting System (FAERS) Database
Abstract
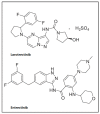
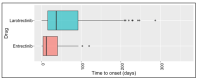
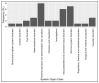
The first-generation tropomyosin receptor kinase (TRK) inhibitors, larotrectinib and entrectinib, represent exciting new developments in cancer treatment that offer relevant, rapid, and long-lasting clinical benefits. Larotrectinib and entrectinib are recommended as first-line treatments for locally advanced or metastatic non-small cell lung cancer (NSCLC) patients with positive TRK gene fusions. In this study, using the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database between 2019 and 2022, a retrospective analysis was conducted to evaluate the safety profiles of these drugs. During our study period, 807 individual case safety reports (ICSRs) related to larotrectinib or entrectinib were retrieved from the FAERS database, of which 48.7% referred to females and 24.7% referred to adult patients (18-64 years) with a median age of 61.0 years. A total of 1728 adverse drug reactions (ADRs) were identified. The most frequently reported ADRs were dizziness and pain, which belong to the System Organ Classes (SOCs) "nervous system disorders" and "general disorders and administration site conditions". Regarding all ADRs, the median time to onset was 37.0 days for larotrectinib and 12.0 days for entrectinib. No evident safety concerns emerged in the long-term safety profiles (>365 days). Only 18 ICSRs were related to pediatric populations (≤16 years), of which 94.0% of the ICSRs were related to larotrectinib. The median age was 10.5 years, while most patients were female (44.4%). Our results show favorable risk-benefit profiles for larotrectinib and entrectinib. Considering the increased use of neurotrophic tyrosine receptor kinase (NTRK) inhibitors, continuous safety monitoring of larotrectinib and entrectinib is required for the detection of possible new adverse drug reactions.
Keywords: NTRK; TRK; agnostic drugs; entrectinib; larotrectinib; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

