Pharmacomicrobiomics of Classical Immunosuppressant Drugs: A Systematic Review
- PMID: 37761003
- PMCID: PMC10526314
- DOI: 10.3390/biomedicines11092562
Pharmacomicrobiomics of Classical Immunosuppressant Drugs: A Systematic Review
Abstract
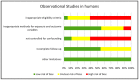
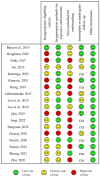
The clinical response to classical immunosuppressant drugs (cIMDs) is highly variable among individuals. We performed a systematic review of published evidence supporting the hypothesis that gut microorganisms may contribute to this variability by affecting cIMD pharmacokinetics, efficacy or tolerability. The evidence that these drugs affect the composition of intestinal microbiota was also reviewed. The PubMed and Scopus databases were searched using specific keywords without limits of species (human or animal) or time from publication. One thousand and fifty five published papers were retrieved in the initial database search. After screening, 50 papers were selected to be reviewed. Potential effects on cIMD pharmacokinetics, efficacy or tolerability were observed in 17/20 papers evaluating this issue, in particular with tacrolimus, cyclosporine, mycophenolic acid and corticosteroids, whereas evidence was missing for everolimus and sirolimus. Only one of the papers investigating the effect of cIMDs on the gut microbiota reported negative results while all the others showed significant changes in the relative abundance of specific intestinal bacteria. However, no unique pattern of microbiota modification was observed across the different studies. In conclusion, the available evidence supports the hypothesis that intestinal microbiota could contribute to the variability in the response to some cIMDs, whereas data are still missing for others.
Keywords: corticosteroids; cyclosporine; everolimus; methylprednisolone; microbiota; mycophenolic acid; prednisolone; prednisone; sirolimus; tacrolimus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

