Precision Medicine in Pancreatic Ductal Adenocarcinoma: The Impact of Targeted Therapies on Survival of Patients Harboring Actionable Mutations
- PMID: 37761010
- PMCID: PMC10526242
- DOI: 10.3390/biomedicines11092569
Precision Medicine in Pancreatic Ductal Adenocarcinoma: The Impact of Targeted Therapies on Survival of Patients Harboring Actionable Mutations
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of death by cancer worldwide. Mostly diagnosed with locally advanced or metastatic disease, patients lack treatment options. Gene alterations (GAs) are frequently observed in PDAC, some of which are considered for molecular targeted therapies (MTTs), with potential clinical benefits and improved outcomes. However, the applicability of molecular profiling (MP) for precision medicine in PDAC remains to be demonstrated.
Methods: We conducted a retrospective analysis of all patients, aged ≥18 years with histologically confirmed PDAC, who underwent tumor MP between 2010 and 2020 in our institution as part of personalized medicine trials. The primary study endpoint was overall survival (OS), and (minimal follow-up was 6 months after MP).
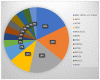
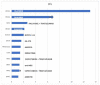
Results: Of 115 eligible patients, MP was successful in 102 patients (89%). KRAS mutations were the most frequent GAs, mostly G12D. Based on ESCAT classification, actionable GAs were found in 29 patients (28%), involving mainly BRCA1 or BRCA2 (5 (18%)), HER2 (5 (18%)), MTAP (5 (18%)), and FGFR (3 (11%)). Only 12 of these 29 patients (41%, or 10% of the whole population) received MTTs, with a median progression-free survival of 1.6 months. Median OS was 19 months in patients with actionable GAs treated with MTTs (n = 12 (11.8%)), 14 months in patients with actionable GAs treated with standard therapies (n = 17 (16.7%)), and 17 months in patients without actionable GAs treated with standard therapies (n = 73 (71.5%); p = 0.26). The absence of liver metastases was associated with better OS (HR = 0.471, p = 0.01). The highest OS following MTT was observed in patients with BRCA mutations treated with olaparib.
Interpretation: Actionable GAs were found in more than a quarter of patients with advanced PDAC. Overall, targeting actionable GAs with MTTs was not associated with improved OS in this retrospective study with limited patient numbers. However, selected GA/MTT combinations (e.g., BRCA mutations/olaparib) were associated with a better outcome.
Keywords: PDAC; gene alterations; molecular profiling; molecular targeted therapies; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

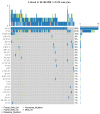

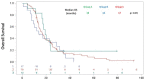
 ongoing treatment; † dead). Better PFS is seen in patients with BRCA2 mutations that were treated with olaparib.
ongoing treatment; † dead). Better PFS is seen in patients with BRCA2 mutations that were treated with olaparib.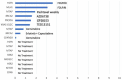
Similar articles
-
Olaparib Monotherapy for Previously Treated Pancreatic Cancer With DNA Damage Repair Genetic Alterations Other Than Germline BRCA Variants: Findings From 2 Phase 2 Nonrandomized Clinical Trials.JAMA Oncol. 2021 May 1;7(5):693-699. doi: 10.1001/jamaoncol.2021.0006. JAMA Oncol. 2021. PMID: 33662100 Free PMC article. Clinical Trial.
-
Alterations in driver genes are predictive of survival in patients with resected pancreatic ductal adenocarcinoma.Cancer. 2020 Sep 1;126(17):3939-3949. doi: 10.1002/cncr.33038. Epub 2020 Jun 23. Cancer. 2020. PMID: 32573775 Free PMC article.
-
Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial.Lancet Oncol. 2020 Apr;21(4):508-518. doi: 10.1016/S1470-2045(20)30074-7. Epub 2020 Mar 2. Lancet Oncol. 2020. PMID: 32135080 Free PMC article.
-
Recent advances in precision medicine for pancreatic ductal adenocarcinoma.Ann Gastroenterol Surg. 2021 Feb 3;5(4):457-466. doi: 10.1002/ags3.12436. eCollection 2021 Jul. Ann Gastroenterol Surg. 2021. PMID: 34337294 Free PMC article. Review.
-
Targeting DNA damage repair pathways in pancreas cancer.Cancer Metastasis Rev. 2021 Sep;40(3):891-908. doi: 10.1007/s10555-021-09983-1. Epub 2021 Aug 17. Cancer Metastasis Rev. 2021. PMID: 34403012 Free PMC article. Review.
Cited by
-
Role of molecular biology in the management of pancreatic cancer.World J Gastrointest Oncol. 2024 Jul 15;16(7):2902-2914. doi: 10.4251/wjgo.v16.i7.2902. World J Gastrointest Oncol. 2024. PMID: 39072173 Free PMC article. Review.
References
-
- Goldstein D., El-Maraghi R.H., Hammel P., Heinemann V., Kunzmann V., Sastre J., Scheithauer W., Siena S., Tabernero J., Teixeira L., et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015;107:dju413. doi: 10.1093/jnci/dju413. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

