Thermal Desorption of 2,4,6-Trichloroanisole from Cork
- PMID: 37761159
- PMCID: PMC10529625
- DOI: 10.3390/foods12183450
Thermal Desorption of 2,4,6-Trichloroanisole from Cork
Abstract
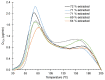
While extensive efforts have been made over the past two decades to understand how cork becomes contaminated by 2,4,6-trichloroanisole (TCA), the nature of its bond to cork remains unclear. A deeper understanding of this interaction is crucial in designing processes to effectively remove TCA from cork stoppers. This study presents an investigation into the thermal desorption of TCA from cork under vacuum conditions. To facilitate detection by a quadrupole mass spectrometer, samples were artificially contaminated with sufficient TCA. A calibration system was developed to determine the absolute rate of TCA released from the cork. Desorption spectra revealed two peaks at 80 °C and 170 °C. Despite the known variability of cork, repeated measurements demonstrated reasonable repeatability. The low-temperature peak decreased with time and after preheating the sample to 50 °C. It is proposed that the high-temperature peak corresponds to TCA bonded to the cork material. Experiments with naturally contaminated cork stoppers revealed a significant reduction in the amount of releasable TCA following a vacuum-heating process. This study provides an insightful discussion on the adsorption of TCA on cork and proposes an estimate for the adsorption energy. Furthermore, it discloses a process capable of removing TCA from natural cork stoppers.
Keywords: TCA; TCA extraction; calibration method; cork stoppers; mass spectrometry; vacuum.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Authors Susana Monteiro, Paulo Lopes and Miguel Cabral were employed by the company Amorim Cork S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from the Portuguese Foundation for Science and Technology (FCT). The funder was not involved in the study, design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. Nova School of Sciences and Technology holds two patents (# WO 2018/138599 A1 and #PT109878B) authored by Orlando Manuel Neves Duarte Teodoro with royalties paid by Amorim Cork S.A.
Figures





References
-
- Buser H.-R.R., Zanier C., Tanner H. Identification of 2,4,6-Trichloroanisole as a potent compound causing cork taint in wine. J. Agric. Food Chem. 1982;30:359–362. doi: 10.1021/jf00110a037. - DOI
-
- Sefton M.A., Simpson R.F. Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine—A review. Aust. J. Grape Wine Res. 2005;11:226–240. doi: 10.1111/j.1755-0238.2005.tb00290.x. - DOI
-
- Álvarez-Rodríguez M.L., López-Ocaña L., López-Coronado J.M., Rodríguez E., Martínez M.J., Larriba G., Coque J.-J.R. Cork Taint of Wines: Role of the Filamentous Fungi Isolated from Cork in the Formation of 2,4,6-Trichloroanisole by O Methylation of 2,4,6-Trichlorophenol. Appl. Environ. Microbiol. 2002;68:5860–5869. doi: 10.1128/AEM.68.12.5860-5869.2002. - DOI - PMC - PubMed
-
- Barker D.A., Capone D.L., Pollnitz A.P., McLean H.J., Francis I.L., Oakey H., Sefton M.A. Absorption of 2,4,6-trichloroanisole by wine corks via the vapour phase in an enclosed environment. Aust. J. Grape Wine Res. 2001;7:40–46. doi: 10.1111/j.1755-0238.2001.tb00192.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

