Evaluating the Use of PROMs in Paediatric Orthopaedic Registries
- PMID: 37761513
- PMCID: PMC10528097
- DOI: 10.3390/children10091552
Evaluating the Use of PROMs in Paediatric Orthopaedic Registries
Abstract
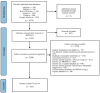
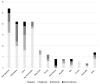
Patient-reported outcome measures (PROMs) provide structured information on the patient's health experience and facilitate shared clinical decision-making. Registries that collect PROMs generate essential information about the clinical course and efficacy of interventions. Whilst PROMs are increasingly being used in adult orthopaedic registries, their use in paediatric orthopaedic registries is not well known. The purpose of this systematic review was to identify the frequency and scope of registries that collect PROMs in paediatric orthopaedic patient groups. In July 2023, six databases were systematically searched to identify studies that collected PROMs using a registry amongst patients aged under 18 years with orthopaedic diagnoses. Of 3190 identified articles, 128 unique registries were identified. Three were exclusively paediatric, 27 were majority paediatric, and the remainder included a minority of paediatric patients. One hundred and twenty-eight registries collected 72 different PROMs, and 58% of these PROMs were not validated for a paediatric population. The largest group of orthopaedic registries collected PROMs on knee ligament injuries (21%). There are few reported dedicated orthopaedic registries collecting PROMs in paediatric populations. The majority of PROMs collected amongst paediatric populations by orthopaedic registries are not validated for patients under the age of 18 years. The use of non-validated PROMs by registries greatly impedes their utility and impact. Dedicated orthopaedic registries collecting paediatric-validated PROMs are needed to increase health knowledge, improve decision-making between patients and healthcare providers, and optimise orthopaedic management.
Keywords: PROMs; orthopaedic; paediatric; registry; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fleischmann M., Vaughan B. The challenges and opportunities of using patient reported outcome measures (PROMs) in clinical practice. Int. J. Osteopat. Med. 2018;28:56–61. doi: 10.1016/j.ijosm.2018.03.003. - DOI
-
- Rolfson O., Bohm E., Franklin P., Lyman S., Denissen G., Dawson J., Dunn J., Chenok K.E., Dunbar M., Overgaard S., et al. Patient-reported outcome measures in arthroplasty registries. Report of the Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries Part II. Recommendations for selection, administration, and analysis. Acta Orthop. 2016;87:9–23. doi: 10.1080/17453674.2016.1181816. - DOI - PMC - PubMed
-
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources