Heterozygosity of ALG9 in Association with Autosomal Dominant Polycystic Liver Disease
- PMID: 37761895
- PMCID: PMC10530326
- DOI: 10.3390/genes14091755
Heterozygosity of ALG9 in Association with Autosomal Dominant Polycystic Liver Disease
Abstract
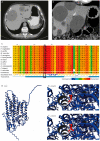
α-1,2-mannosyltransferase (ALG9) germline variants are linked to autosomal dominant polycystic kidney disease (ADPKD). Many individuals affected with ADPKD possess polycystic livers as a common extrarenal manifestation. We performed whole exome sequencing in a female with autosomal dominant polycystic liver disease (ADPLD) without kidney cysts and established the presence of a heterozygous missense variant (c.677G>C p.(Gly226Ala)) in ALG9. In silico pathogenicity prediction and 3D protein modeling determined this variant as pathogenic. Loss of heterozygosity is regularly seen in liver cyst walls. Immunohistochemistry indicated the absence of ALG9 in liver tissue from this patient. ALG9 expression was absent in cyst wall lining from ALG9- and PRKCSH-caused ADPLD patients but present in the liver cyst lining derived from an ADPKD patient with a PKD2 variant. Thus, heterozygous pathogenic variants in ALG9 are also associated with ADPLD. Somatic loss of heterozygosity of the ALG9 enzyme was seen in the ALG9 patient but also in ADPLD patients with a different genetic background. This expanded the phenotypic spectrum of ADPLD to ALG9.
Keywords: ADPLD; ALG9; PCLD; cyst wall; polycystic liver disease (PLD); whole exome sequencing.
Conflict of interest statement
The Radboudumc, on behalf of J.P.H.D., received research grants from Gilead and Abbvie for unrelated research. He is the PI of the POSITANO study (Camurus). The funder, Radboud Institute for Molecular Life Sciences/Research Institute for Medical Innovation, did not have input on the research and content in this manuscript. The remaining authors report no conflict of interest.
Figures

References
-
- Tham E., Eklund E.A., Hammarsjö A., Bengtson P., Geiberger S., Lagerstedt-Robinson K., Malmgren H., Nilsson D., Grigelionis G., Conner P., et al. A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach-Nishimura skeletal dysplasia due to pathogenic variants in ALG9. Eur. J. Hum. Genet. 2016;24:198–207. doi: 10.1038/ejhg.2015.91. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

