Phenomic Microglia Diversity as a Druggable Target in the Hippocampus in Neurodegenerative Diseases
- PMID: 37761971
- PMCID: PMC10531074
- DOI: 10.3390/ijms241813668
Phenomic Microglia Diversity as a Druggable Target in the Hippocampus in Neurodegenerative Diseases
Abstract
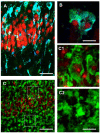
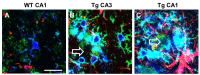
Phenomics, the complexity of microglia phenotypes and their related functions compels the continuous study of microglia in disease animal models to find druggable targets for neurodegenerative disorders. Activation of microglia was long considered detrimental for neuron survival, but more recently it has become apparent that the real scenario of microglia morphofunctional diversity is far more complex. In this review, we discuss the recent literature on the alterations in microglia phenomics in the hippocampus of animal models of normal brain aging, acute neuroinflammation, ischemia, and neurodegenerative disorders, such as AD. Microglia undergo phenomic changes consisting of transcriptional, functional, and morphological changes that transform them into cells with different properties and functions. The classical subdivision of microglia into M1 and M2, two different, all-or-nothing states is too simplistic, and does not correspond to the variety of phenotypes recently discovered in the brain. We will discuss the phenomic modifications of microglia focusing not only on the differences in microglia reactivity in the diverse models of neurodegenerative disorders, but also among different areas of the brain. For instance, in contiguous and highly interconnected regions of the rat hippocampus, microglia show a differential, finely regulated, and region-specific reactivity, demonstrating that microglia responses are not uniform, but vary significantly from area to area in response to insults. It is of great interest to verify whether the differences in microglia reactivity may explain the differential susceptibility of different brain areas to insults, and particularly the higher sensitivity of CA1 pyramidal neurons to inflammatory stimuli. Understanding the spatiotemporal heterogeneity of microglia phenomics in health and disease is of paramount importance to find new druggable targets for the development of novel microglia-targeted therapies in different CNS disorders. This will allow interventions in three different ways: (i) by suppressing the pro-inflammatory properties of microglia to limit the deleterious effect of their activation; (ii) by modulating microglia phenotypic change to favor anti-inflammatory properties; (iii) by influencing microglia priming early in the disease process.
Keywords: Alzheimer’s disease; LPS inflammation; TRPV2 channels; cannabidiol; inflammaging; ischemia; microbiota; reactive microglia; α7nACh receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
- DN. 1553 11.10.2022/#NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - A Multiscale in-tegrated approach to the study of the nervous system in health and disease
- PROPREBIOAD/Fondazione Cassa di Risparmio Firenze
- giovanniniricaten2022/Ministero dell'Istruzione, dell'Università e della Ricerca
- Post-doctoral Fellowships 2023/Fondazione U. Veronesi
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

