Anti-Inflammatory Effects of Miyako Bidens pilosa in a Mouse Model of Amyotrophic Lateral Sclerosis and Lipopolysaccharide-Stimulated BV-2 Microglia
- PMID: 37762010
- PMCID: PMC10530530
- DOI: 10.3390/ijms241813698
Anti-Inflammatory Effects of Miyako Bidens pilosa in a Mouse Model of Amyotrophic Lateral Sclerosis and Lipopolysaccharide-Stimulated BV-2 Microglia
Abstract
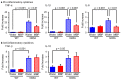
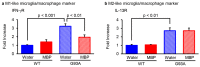
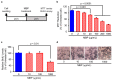
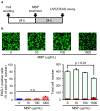
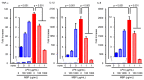
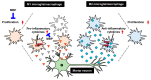
Neuroinflammation is a fundamental feature in the pathogenesis of amyotrophic lateral sclerosis (ALS) and arises from the activation of astrocytes and microglial cells. Previously, we reported that Miyako Bidens pilosa extract (MBP) inhibited microglial activation and prolonged the life span in a human ALS-linked mutant superoxide dismutase-1 (SOD1G93A) transgenic mouse model of ALS (G93A mice). Herein, we evaluated the effect of MBP on microglial activation in the spinal cord of G93A mice and lipopolysaccharide-stimulated BV-2 microglial cells. The administration of MBP inhibited the upregulation of the M1-microglia/macrophage marker (interferon-γ receptor (IFN-γR)) and pro-inflammatory cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6) in G93A mice. However, MBP did not affect the increase in the M2-microglia/macrophage marker (IL-13R) and anti-inflammatory cytokines (transforming growth factor (TGF)-β and IL-10) in G93A mice. BV-2 cell exposure to MBP resulted in a decrease in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) reduction activity and bromodeoxyuridine incorporation, without an increase in the number of ethidium homodimer-1-stained dead cells. Moreover, MBP suppressed the production of lipopolysaccharide-induced pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in BV-2 cells. These results suggest that the selective suppression of M1-related pro-inflammatory cytokines is involved in the therapeutic potential of MBP in ALS model mice.
Keywords: Bidens pilosa; M1-microglia/macrophages; amyotrophic lateral sclerosis; pro-inflammatory cytokines; spinal cord.
Conflict of interest statement
Yasuhiro Kosuge had grants from Refine Holdings Co., Ltd., and the other authors declare no competing financial interest.
Figures
Similar articles
-
Bidens pilosa Extract Administered after Symptom Onset Attenuates Glial Activation, Improves Motor Performance, and Prolongs Survival in a Mouse Model of Amyotrophic Lateral Sclerosis.Oxid Med Cell Longev. 2020 Jan 29;2020:1020673. doi: 10.1155/2020/1020673. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32089764 Free PMC article.
-
Ablation of interleukin-19 improves motor function in a mouse model of amyotrophic lateral sclerosis.Mol Brain. 2021 Apr 30;14(1):74. doi: 10.1186/s13041-021-00785-8. Mol Brain. 2021. PMID: 33931083 Free PMC article.
-
Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article.
-
Neuroinflammation and Amyotrophic Lateral Sclerosis: Recent Advances in Anti-Inflammatory Cytokines as Therapeutic Strategies.Int J Mol Sci. 2025 Apr 18;26(8):3854. doi: 10.3390/ijms26083854. Int J Mol Sci. 2025. PMID: 40332510 Free PMC article. Review.
-
Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study.Sci Rep. 2017 Aug 22;7(1):9094. doi: 10.1038/s41598-017-09097-1. Sci Rep. 2017. PMID: 28831083 Free PMC article.
Cited by
-
Miyako Bidens pilosa Extract Ameliorates Allodynia and Suppresses Spinal Microglial Activation in Mice with Partial Sciatic Nerve Ligation.Curr Issues Mol Biol. 2025 Jun 12;47(6):453. doi: 10.3390/cimb47060453. Curr Issues Mol Biol. 2025. PMID: 40699852 Free PMC article.
-
Exploring the phytochemical profile, antioxidant and anti-inflammatory potential of Bidens pilosa: A Systematic Review.Front Pharmacol. 2025 Aug 1;16:1569527. doi: 10.3389/fphar.2025.1569527. eCollection 2025. Front Pharmacol. 2025. PMID: 40822453 Free PMC article.
-
Functional metabolomics: unlocking the role of small molecular metabolites.Front Mol Biosci. 2025 Jul 9;12:1542100. doi: 10.3389/fmolb.2025.1542100. eCollection 2025. Front Mol Biosci. 2025. PMID: 40703706 Free PMC article. Review.
-
Effect of β-1,4-GalTI on the biological function of astrocytes treated by LPS.Biomol Biomed. 2024 Dec 11;25(1):226-239. doi: 10.17305/bb.2024.11088. Biomol Biomed. 2024. PMID: 39284278 Free PMC article.
-
Temporal changes of spinal microglia in murine models of neuropathic pain: a scoping review.Front Immunol. 2024 Dec 6;15:1460072. doi: 10.3389/fimmu.2024.1460072. eCollection 2024. Front Immunol. 2024. PMID: 39735541 Free PMC article.
References
-
- Miana-Mena F.J., González-Mingot C., Larrodé P., Muñoz M.J., Oliván S., Fuentes-Broto L., Martínez-Ballarín E., Reiter R.J., Osta R., García J.J. Monitoring Systemic Oxidative Stress in an Animal Model of Amyotrophic Lateral Sclerosis. J. Neurol. 2011;258:762–769. doi: 10.1007/s00415-010-5825-8. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

