Euphorbiasteroid Induces Apoptosis as Well as Autophagy through Modulating SHP-1/STAT3 Pathway in Hepatocellular Carcinoma Cells
- PMID: 37762016
- PMCID: PMC10531000
- DOI: 10.3390/ijms241813713
Euphorbiasteroid Induces Apoptosis as Well as Autophagy through Modulating SHP-1/STAT3 Pathway in Hepatocellular Carcinoma Cells
Abstract
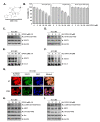
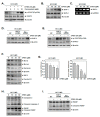
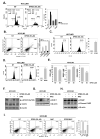
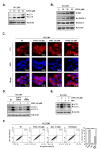
Euphorbiasteroid (EPBS) has gained attention for its activity against human lung cancer and sarcoma; however, its impact on hepatocellular carcinoma has not yet been elucidated. Here, we investigated the cytotoxic effect of EPBS on human hepatocellular carcinoma (HCC) cells. We found that EPBS induced both apoptosis and autophagy in HCC cells. Additionally, we observed that EPBS treatment suppressed the constitutive as well as the inducible activation of a signal transducer and activator of transcription 3 (STAT3) protein expression. Moreover, EPBS promoted the expression of SHP-1 protein and the production of reactive oxidative stress (ROS). Furthermore, the knockdown of SHP-1 by siRNA transfection reversed the effects of EPBS, which have inductive effects related to apoptosis and autophagy. Therefore, EPBS can potentially function as an anti-cancer agent by inducing apoptosis and autophagy when targeting the SHP-1/STAT3 pathway.
Keywords: SHP-1; STAT3; apoptosis; autophagy; euphorbiasteroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
3-Formylchromone Counteracts STAT3 Signaling Pathway by Elevating SHP-2 Expression in Hepatocellular Carcinoma.Biology (Basel). 2021 Dec 26;11(1):29. doi: 10.3390/biology11010029. Biology (Basel). 2021. PMID: 35053027 Free PMC article.
-
A traditional prescription comprising Astragali Radix and Schisandra chinensis Fructus induces apoptosis and protective autophagy in hepatocellular carcinoma cells.J Ethnopharmacol. 2023 Aug 10;312:116548. doi: 10.1016/j.jep.2023.116548. Epub 2023 Apr 24. J Ethnopharmacol. 2023. PMID: 37100264
-
Negative regulation of signal transducer and activator of transcription-3 signalling cascade by lupeol inhibits growth and induces apoptosis in hepatocellular carcinoma cells.Br J Cancer. 2014 Sep 23;111(7):1327-37. doi: 10.1038/bjc.2014.422. Epub 2014 Aug 7. Br J Cancer. 2014. PMID: 25101566 Free PMC article.
-
Dovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3.Mol Cancer Ther. 2012 Feb;11(2):452-63. doi: 10.1158/1535-7163.MCT-11-0412. Epub 2011 Dec 16. Mol Cancer Ther. 2012. PMID: 22180308
-
Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1.J Cell Physiol. 2012 May;227(5):2184-95. doi: 10.1002/jcp.22954. J Cell Physiol. 2012. PMID: 21792937
Cited by
-
Costunolide inhibits the progression of TPA-induced cell transformation and DMBA/TPA-induced skin carcinogenesis by regulation of AKT-mediated signaling.Cancer Cell Int. 2025 Mar 20;25(1):106. doi: 10.1186/s12935-025-03742-w. Cancer Cell Int. 2025. PMID: 40114176 Free PMC article.
-
A new 1,2,3-triazole-indirubin hybrid suppresses tumor growth and pulmonary metastasis by mitigating the HGF/c-MET axis in hepatocellular carcinoma.J Adv Res. 2025 Jul;73:341-356. doi: 10.1016/j.jare.2024.08.033. Epub 2024 Aug 30. J Adv Res. 2025. PMID: 39216686 Free PMC article.
-
Oxazine drug-seed induces paraptosis and apoptosis through reactive oxygen species/JNK pathway in human breast cancer cells.Transl Oncol. 2024 Nov;49:102101. doi: 10.1016/j.tranon.2024.102101. Epub 2024 Aug 18. Transl Oncol. 2024. PMID: 39159553 Free PMC article.
References
-
- Hashemi M., Sabouni E., Rahmanian P., Entezari M., Mojtabavi M., Raei B., Zandieh M.A., Behroozaghdam M., Mirzaei S., Hushmandi K., et al. Deciphering STAT3 signaling potential in hepatocellular carcinoma: Tumorigenesis, treatment resistance, and pharmacological significance. Cell Mol. Biol. Lett. 2023;28:33. doi: 10.1186/s11658-023-00438-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

