Transcriptome Signature of Immature and In Vitro-Matured Equine Cumulus-Oocytes Complex
- PMID: 37762020
- PMCID: PMC10531358
- DOI: 10.3390/ijms241813718
Transcriptome Signature of Immature and In Vitro-Matured Equine Cumulus-Oocytes Complex
Abstract
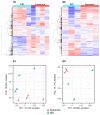
Maturation is a critical step in the development of an oocyte, and it is during this time that the oocyte advances to metaphase II (MII) of the meiotic cycle and acquires developmental competence to be fertilized and become an embryo. However, in vitro maturation (IVM) remains one of the limiting steps in the in vitro production of embryos (IVP), with a variable percentage of oocytes reaching the MII stage and unpredictable levels of developmental competence. Understanding the dynamics of oocyte maturation is essential for the optimization of IVM culture conditions and subsequent IVP outcomes. Thus, the aim of this study was to elucidate the transcriptome dynamics of oocyte maturation by comparing transcriptomic changes during in vitro maturation in both oocytes and their surrounding cumulus cells. Cumulus-oocyte complexes were obtained from antral follicles and divided into two groups: immature and in vitro-matured (MII). RNA was extracted separately from oocytes (OC) and cumulus cells (CC), followed by library preparation and RNA sequencing. A total of 13,918 gene transcripts were identified in OC, with 538 differentially expressed genes (DEG) between immature OC and in vitro-matured OC. In CC, 13,104 genes were expressed with 871 DEG. Gene ontology (GO) analysis showed an association between the DEGs and pathways relating to nuclear maturation in OC and GTPase activity, extracellular matrix organization, and collagen trimers in CC. Additionally, the follicle-stimulating hormone receptor gene (FSHR) and luteinizing hormone/choriogonadotropin receptor gene (LHCGR) showed differential expressions between CC-MII and immature CC samples. Overall, these results serve as a foundation to further investigate the biological pathways relevant to oocyte maturation in horses and pave the road to improve the IVP outcomes and the overall clinical management of equine assisted reproductive technologies (ART).
Keywords: cumulus cell; equine; in vitro embryo production; in vitro oocyte maturation; oocyte; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production.Mol Hum Reprod. 2016 Dec;22(12):882-897. doi: 10.1093/molehr/gaw055. Epub 2016 Aug 24. Mol Hum Reprod. 2016. PMID: 27559149
-
Assessment of active translation in cumulus-enclosed and denuded oocytes during standard in vitro maturation and early embryo development.Hum Reprod. 2024 Aug 1;39(8):1752-1766. doi: 10.1093/humrep/deae126. Hum Reprod. 2024. PMID: 38876973
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Transcriptomic profiles reveal the characteristics of oocytes and cumulus cells at GV, MI, and MII in follicles before ovulation.J Ovarian Res. 2023 Nov 22;16(1):225. doi: 10.1186/s13048-023-01291-2. J Ovarian Res. 2023. PMID: 37993893 Free PMC article. Review.
-
Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence.Hum Reprod Update. 2021 Jan 4;27(1):27-47. doi: 10.1093/humupd/dmaa043. Hum Reprod Update. 2021. PMID: 33020823 Review.
References
-
- Galli C., Colleoni S., Duchi R., Lagutina I., Lazzari G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 2007;98:39–55. doi: 10.1016/j.anireprosci.2006.10.011. - DOI - PubMed
-
- Galli C., Duchi R., Colleoni S., Lagutina I., Lazzari G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology. 2014;81:138–151. doi: 10.1016/j.theriogenology.2013.09.008. - DOI - PubMed

