The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation?
- PMID: 37762052
- PMCID: PMC10531172
- DOI: 10.3390/ijms241813750
The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation?
Abstract
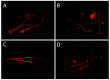
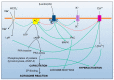
The main cation/calcium channel of spermatozoa (CatSper), first identified in 2001, has been thoroughly studied to elucidate its composition and function, while its distribution among species and sperm sources is yet incomplete. CatSper is composed of several subunits that build a pore-forming calcium channel, mainly activated in vivo in ejaculated sperm cells by intracellular alkalinization and progesterone, as suggested by the in vitro examinations. The CatSper channel relevance is dual: to maintain sperm homeostasis (alongside the plethora of membrane channels present) as well as being involved in pre-fertilization events, such as sperm capacitation, hyperactivation of sperm motility and the acrosome reaction, with remarkable species differences. Interestingly, the observed variations in CatSper localization in the plasma membrane seem to depend on the source of the sperm cells explored (i.e., epididymal or ejaculated, immature or mature, processed or not), the method used for examination and, particularly, on the specificity of the antibodies employed. In addition, despite multiple findings showing the relevance of CatSper in fertilization, few studies have studied CatSper as a biomarker to fine-tune diagnosis of sub-fertility in livestock or even consider its potential to control fertilization in plague animals, a more ethically defensible strategy than implicating CatSper to pharmacologically modify male-related fertility control in humans, pets or wild animals. This review describes inter- and intra-species differences in the localization, structure and function of the CatSper channel, calling for caution when considering its potential manipulation for fertility control or improvement.
Keywords: CatSper; biomarker; calcium; livestock; sperm cells; sub-fertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mann T., Lutwak-Mann C. Male Reproductive Function and Semen. 1st ed. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 1981. p. 507.
-
- Vicente Carrillo A. PhD Thesis. Linköping University; Linköping, Sweden: 2016. Sperm Membrane Channels, Receptors and Kinematics: Using Boar Spermatozoa for Drug Toxicity Screening.
Publication types
MeSH terms
Substances
Grants and funding
- 2019-00288/Swedish Research Council for Environment Agricultural Sciences and Spatial Planning
- PID2019-108320RJ-I00/Spanish Ministry of Science and Innovation
- IJCI-2015-24380/Spanish Ministry of Science and Innovation
- RYC2020-028615-I/Spanish Ministry of Science and Innovation
- MCIN/AEI /10.13039/501100011033/Spanish Ministry of Science and Innovation
LinkOut - more resources
Full Text Sources

