A Multiplexed Urinary Biomarker Panel Has Potential for Alzheimer's Disease Diagnosis Using Targeted Proteomics and Machine Learning
- PMID: 37762058
- PMCID: PMC10531486
- DOI: 10.3390/ijms241813758
A Multiplexed Urinary Biomarker Panel Has Potential for Alzheimer's Disease Diagnosis Using Targeted Proteomics and Machine Learning
Abstract
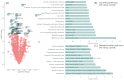
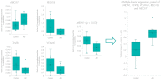
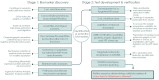
As disease-modifying therapies are now available for Alzheimer's disease (AD), accessible, accurate and affordable biomarkers to support diagnosis are urgently needed. We sought to develop a mass spectrometry-based urine test as a high-throughput screening tool for diagnosing AD. We collected urine from a discovery cohort (n = 11) of well-characterised individuals with AD (n = 6) and their asymptomatic, CSF biomarker-negative study partners (n = 5) and used untargeted proteomics for biomarker discovery. Protein biomarkers identified were taken forward to develop a high-throughput, multiplexed and targeted proteomic assay which was tested on an independent cohort (n = 21). The panel of proteins identified are known to be involved in AD pathogenesis. In comparing AD and controls, a panel of proteins including MIEN1, TNFB, VCAM1, REG1B and ABCA7 had a classification accuracy of 86%. These proteins have been previously implicated in AD pathogenesis. This suggests that urine-targeted mass spectrometry has potential utility as a diagnostic screening tool in AD.
Keywords: Alzheimer’s; biomarkers; diagnosis; machine learning; mass spectrometry; proteomics; urine.
Conflict of interest statement
H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). RWP is co-lead for the Neurofilament light consortium, and industry funded academic collaboration funded by Abbvie, Biogen, Bristol Myers Squibb and Roche.
Figures


References
-
- García-Morales V., González-Acedo A., Melguizo-Rodríguez L., Pardo-Moreno T., Costela-Ruiz V.J., Montiel-Troya M., Ramos-Rodríguez J.J. Current understanding of the physiopathology, diagnosis and therapeutic approach to Alzheimer’s disease. Biomedicines. 2021;9:1910. doi: 10.3390/biomedicines9121910. - DOI - PMC - PubMed
-
- Bridel C., Van Wieringen W.N., Zetterberg H., Tijms B.M., Teunissen C.E., Alvarez-Cermeño J.C., Andreasson U., Axelsson M., Bäckström D.C., Bartos A. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;76:1035–1048. doi: 10.1001/jamaneurol.2019.1534. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

