Brain Hydrophobic Peptides Antagonists of Neurotoxic Amyloid β Peptide Monomers/Oligomers-Protein Interactions
- PMID: 37762148
- PMCID: PMC10531495
- DOI: 10.3390/ijms241813846
Brain Hydrophobic Peptides Antagonists of Neurotoxic Amyloid β Peptide Monomers/Oligomers-Protein Interactions
Abstract
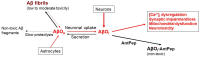
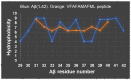
Amyloid β (Aβ) oligomers have been linked to Alzheimer's disease (AD) pathogenesis and are the main neurotoxic forms of Aβ. This review focuses on the following: (i) the Aβ(1-42):calmodulin interface as a model for the design of antagonist Aβ peptides and its limitations; (ii) proteolytic degradation as the major source of highly hydrophobic peptides in brain cells; and (iii) brain peptides that have been experimentally demonstrated to bind to Aβ monomers or oligomers, Aβ fibrils, or Aβ plaques. It is highlighted that the hydrophobic amino acid residues of the COOH-terminal segment of Aβ(1-42) play a key role in its interaction with intracellular protein partners linked to its neurotoxicity. The major source of highly hydrophobic endogenous peptides of 8-10 amino acids in neurons is the proteasome activity. Many canonical antigen peptides bound to the major histocompatibility complex class 1 are of this type. These highly hydrophobic peptides bind to Aβ and are likely to be efficient antagonists of the binding of Aβ monomers/oligomers concentrations in the nanomolar range with intracellular proteins. Also, their complexation with Aβ will protect them against endopeptidases, suggesting a putative chaperon-like physiological function for Aβ that has been overlooked until now. Remarkably, the hydrophobic amino acid residues of Aβ responsible for the binding of several neuropeptides partially overlap with those playing a key role in its interaction with intracellular protein partners that mediates its neurotoxicity. Therefore, these latter neuropeptides are also potential candidates to antagonize Aβ peptides binding to target proteins. In conclusion, the analysis performed in this review points out that hydrophobic endogenous brain neuropeptides could be valuable biomarkers to evaluate the risk of the onset of sporadic AD, as well as for the prognosis of AD.
Keywords: Alzheimer’s disease; amyloid β; calmodulin; canonical antigen peptides; endogenous hydrophobic peptides; neuropeptides; neurotoxicity; proteasome.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
The Relevance of Amyloid β-Calmodulin Complexation in Neurons and Brain Degeneration in Alzheimer's Disease.Int J Mol Sci. 2021 May 7;22(9):4976. doi: 10.3390/ijms22094976. Int J Mol Sci. 2021. PMID: 34067061 Free PMC article. Review.
-
Design and Experimental Evaluation of a Peptide Antagonist against Amyloid β(1-42) Interactions with Calmodulin and Calbindin-D28k.Int J Mol Sci. 2022 Feb 18;23(4):2289. doi: 10.3390/ijms23042289. Int J Mol Sci. 2022. PMID: 35216403 Free PMC article.
-
Accumulation of cellular prion protein within β-amyloid oligomer plaques in aged human brains.Brain Pathol. 2021 Sep;31(5):e12941. doi: 10.1111/bpa.12941. Epub 2021 Feb 23. Brain Pathol. 2021. PMID: 33624334 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Specific Binding of Alzheimer's Aβ Peptides to Extracellular Vesicles.Int J Mol Sci. 2024 Mar 26;25(7):3703. doi: 10.3390/ijms25073703. Int J Mol Sci. 2024. PMID: 38612514 Free PMC article.
References
-
- Gong Y., Chang L., Viola K.L., Lacor P.N., Lambert M.P., Finch C.E., Krafft G.A., Klein W.L. Alzheimer’s Disease-Affected Brain: Presence of Oligomeric Aβ Ligands (ADDLs) Suggests a Molecular Basis for Reversible Memory Loss. Proc. Natl. Acad. Sci. USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

