Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota
- PMID: 37762181
- PMCID: PMC10530649
- DOI: 10.3390/ijms241813877
Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota
Abstract
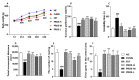
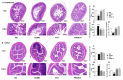
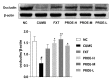
Polygalae radix (PR) is a well-known traditional Chinese medicine that is used to treat depression, and polygalae radix oligosaccharide esters (PROEs) are the main active ingredient. Although gut microbiota are now believed to play key role in depression, the effects of PROEs on depression via modulation of gut microbiota remain unknown. In this article, we investigate the effect of PROEs on the gut microbiota of a depression rat and the possible mechanism responsible. The depression rat model was induced by solitary rearing combined with chronic unpredictable mild stress (CUMS). The depression-like behavior, the influence on the hypothalamic-pituitary-adrenal (HPA) axis, the contents of monoamine neurotransmitter in the hippocampus, and the quantity of short-chain fatty acids (SCFAs) in the feces were each assessed, and the serum levels of lipopolysaccharide (LPS) and interleukin-6 (IL-6) were measured by ELISA. Additionally, ultrastructural changes of the duodenal and colonic epithelium were observed under transmission electron microscope, and the gut microbiota were profiled by using 16S rRNA sequencing. The results show that PROEs alleviated the depression-like behavior of the depression model rats, increased the level of monoamine neurotransmitters in the brain, and reduced the hyperfunction of the HPA axis. Furthermore, PROEs regulated the imbalance of the gut microbiota in the rats, relieving intestinal mucosal damage by increasing the relative abundance of gut microbiota with intestinal barrier protective functions, and adjusting the level of SCFAs in the feces, as well as the serum levels of LPS and IL-6. Thus, we find that PROEs had an antidepressant effect through the restructuring of gut microbiota that restored the function of the intestinal barrier, reduced the release of intestinal endotoxin, and constrained the inflammatory response.
Keywords: chronic unpredictable mild stress; depression; gut microbiota; intestinal barrier; polygalae radix oligosaccharide esters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Total glycosides from stems of Cistanche tubulosa alleviate depression-like behaviors: bidirectional interaction of the phytochemicals and gut microbiota.Phytomedicine. 2021 Mar;83:153471. doi: 10.1016/j.phymed.2021.153471. Epub 2021 Jan 22. Phytomedicine. 2021. PMID: 33636477
-
Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model.Phytomedicine. 2020 Feb;67:153157. doi: 10.1016/j.phymed.2019.153157. Epub 2019 Dec 23. Phytomedicine. 2020. PMID: 31896054
-
Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: intestinal microbiota and gut microbiome function.Food Funct. 2019 Sep 1;10(9):5886-5897. doi: 10.1039/c9fo00399a. Epub 2019 Aug 29. Food Funct. 2019. PMID: 31464319
-
[Gut microbiota and depression : Pathophysiology of depression: hypothalamic-pituitary-adrenal axis and microbiota-gut-brain axis].Nervenarzt. 2020 Dec;91(12):1108-1114. doi: 10.1007/s00115-020-01029-1. Epub 2020 Nov 2. Nervenarzt. 2020. PMID: 33136173 Review. German.
-
Understanding the Connection between Gut Homeostasis and Psychological Stress.J Nutr. 2023 Apr;153(4):924-939. doi: 10.1016/j.tjnut.2023.01.026. Epub 2023 Jan 31. J Nutr. 2023. PMID: 36806451 Review.
Cited by
-
Saponin components in Polygala tenuifolia as potential candidate drugs for treating dementia.Front Pharmacol. 2024 Jul 10;15:1431894. doi: 10.3389/fphar.2024.1431894. eCollection 2024. Front Pharmacol. 2024. PMID: 39050746 Free PMC article. Review.
-
Pathogenesis of depression and the potential for traditional Chinese medicine treatment.Front Pharmacol. 2024 Jun 25;15:1407869. doi: 10.3389/fphar.2024.1407869. eCollection 2024. Front Pharmacol. 2024. PMID: 38983910 Free PMC article. Review.
-
Exploring the interaction between the gut microbiota and cyclic adenosine monophosphate-protein kinase A signaling pathway: a potential therapeutic approach for neurodegenerative diseases.Neural Regen Res. 2025 Nov 1;20(11):3095-3112. doi: 10.4103/NRR.NRR-D-24-00607. Epub 2024 Nov 13. Neural Regen Res. 2025. PMID: 39589173 Free PMC article.
-
Lactitol Alleviates Loperamide-Induced Constipation in Sprague Dawley Rats by Regulating Serotonin, Short-Chain Fatty Acids, and Gut Microbiota.Foods. 2024 Jul 3;13(13):2128. doi: 10.3390/foods13132128. Foods. 2024. PMID: 38998634 Free PMC article.
-
A review of the botany, metabolites, pharmacology, toxicity, industrial applications, and processing of Polygalae Radix: the "key medicine for nourishing life".Front Pharmacol. 2024 Sep 18;15:1450733. doi: 10.3389/fphar.2024.1450733. eCollection 2024. Front Pharmacol. 2024. PMID: 39359244 Free PMC article. Review.
References
-
- Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

