Development of Stable Amino-Pyrimidine-Curcumin Analogs: Synthesis, Equilibria in Solution, and Potential Anti-Proliferative Activity
- PMID: 37762266
- PMCID: PMC10531168
- DOI: 10.3390/ijms241813963
Development of Stable Amino-Pyrimidine-Curcumin Analogs: Synthesis, Equilibria in Solution, and Potential Anti-Proliferative Activity
Abstract
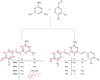
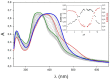
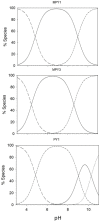
With the clear need for better cancer treatment, naturally occurring molecules represent a powerful inspiration. Recently, curcumin has attracted attention for its pleiotropic anticancer activity in vitro, especially against colorectal and prostate cancer cells. Unfortunately, these encouraging results were disappointing in vivo due to curcumin's low stability and poor bioavailability. To overcome these issues, herein, the synthesis of eight new pyrimidine-curcumin derivatives is reported. The compounds were fully characterized (1H/13C NMR (Nuclear Magnetic Resonance), LC-MS (Liquid Chromatography-Mass Spectrometri), UV-Vis spectroscopy), particularly their acid/base behavior; overall protonation constants were estimated, and species distribution, as a function of pH, was predicted, suggesting that all the compounds are in their neutral form at pH 7.4. All the compounds were extremely stable in simulated physiological media (phosphate-buffered saline and simulated plasma). The compounds were tested in vitro (48 h incubation treatment) to assess their effect on cell viability in prostate cancer (LNCaP and PC3) and colorectal cancer (HT29 and HCT116) cell lines. Two compounds showed the same anti-proliferative activity as curcumin against HCT116 cells and improved cytotoxicity against PC3 cells.
Keywords: cancer cells; cell proliferation; curcumin; pyrimidine derivatives; solution equilibria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- [(accessed on 8 August 2023)]. Available online: https://gco.iarc.fr/today/home.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

