The Effect of Antimicrobial Photodynamic Inactivation on the Protein Profile of Dormant Mycolicibacterium smegmatis Containing Endogenous Porphyrins
- PMID: 37762271
- PMCID: PMC10531400
- DOI: 10.3390/ijms241813968
The Effect of Antimicrobial Photodynamic Inactivation on the Protein Profile of Dormant Mycolicibacterium smegmatis Containing Endogenous Porphyrins
Abstract
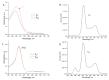
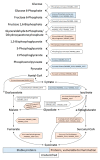
During transition into a dormant state, Mycolicibacterium (Mycobacterium) smegmatis cells are able to accumulate free porphyrins that makes them sensitive to photodynamic inactivation (PDI). The formation of dormant cells in a liquid medium with an increased concentration of magnesium (up to 25 mM) and zinc (up to 62 µM) resulted in an increase in the total amount of endogenous porphyrins in dormant M. smegmatis cells and their photosensitivity, especially for bacteria phagocytosed by macrophages. To gain insight into possible targets for PDI in bacterial dormant mycobacterial cells, a proteomic profiling with SDS gel electrophoresis and mass spectrometry analysis were conducted. Illumination of dormant forms of M. smegmatis resulted in the disappearance of proteins in the separating SDS gel. Dormant cells obtained under an elevated concentration of metal ions were more sensitive to PDI. Differential analysis of proteins with their identification with MALDI-TOF revealed that 45.2% and 63.9% of individual proteins disappeared from the separating gel after illumination for 5 and 15 min, respectively. Light-sensitive proteins include enzymes belonging to the glycolytic pathway, TCA cycle, pentose phosphate pathway, oxidative phosphorylation and energy production. Several proteins involved in protecting against oxygen stress and protein aggregation were found to be sensitive to light. This makes dormant cells highly vulnerable to harmful factors during a long stay in a non-replicative state. PDI caused inhibition of the respiratory chain activity and destroyed enzymes involved in the synthesis of proteins and nucleic acids, the processes which are necessary for dormant cell reactivation and their transition to multiplying bacteria. Because of such multiple targeting, PDI action via endogenous porphyrins could be considered as an effective approach for killing dormant bacteria and a perspective to inactivate dormant mycobacteria and combat the latent form of mycobacteriosis, first of all, with surface localization.
Keywords: Mycobacterium smegmatis; dormant mycobacteria; photodynamic inactivation; porphyrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Photoinactivation of dormant Mycobacterium smegmatis due to its endogenous porphyrins.Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9687-9695. doi: 10.1007/s00253-019-10197-3. Epub 2019 Nov 12. Appl Microbiol Biotechnol. 2019. PMID: 31713670
-
The accumulation of methylated porphyrins in dormant cells of Mycolicibacterium smegmatis is accompanied by a decrease in membrane fluidity and an impede of the functioning of the respiratory chain.Biochim Biophys Acta Biomembr. 2024 Mar;1866(3):184270. doi: 10.1016/j.bbamem.2024.184270. Epub 2024 Jan 9. Biochim Biophys Acta Biomembr. 2024. PMID: 38211647
-
Metabolic profiling of dormant Mycolicibacterium smegmatis cells' reactivation reveals a gradual assembly of metabolic processes.Metabolomics. 2020 Feb 6;16(2):24. doi: 10.1007/s11306-020-1645-8. Metabolomics. 2020. PMID: 32025943
-
Photoinactivation of mycobacteria to combat infection diseases: current state and perspectives.Appl Microbiol Biotechnol. 2021 May;105(10):4099-4109. doi: 10.1007/s00253-021-11349-0. Epub 2021 May 17. Appl Microbiol Biotechnol. 2021. PMID: 33997929 Free PMC article. Review.
-
Hypobiosis of Mycobacteria: Biochemical Aspects.Biochemistry (Mosc). 2023 Jan;88(Suppl 1):S52-S74. doi: 10.1134/S0006297923140043. Biochemistry (Mosc). 2023. PMID: 37069114 Review.
Cited by
-
Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance.Int J Mol Sci. 2025 Aug 19;26(16):7993. doi: 10.3390/ijms26167993. Int J Mol Sci. 2025. PMID: 40869313 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

