Modulation of LPS-Induced Neurodegeneration by Intestinal Helminth Infection in Ageing Mice
- PMID: 37762297
- PMCID: PMC10530578
- DOI: 10.3390/ijms241813994
Modulation of LPS-Induced Neurodegeneration by Intestinal Helminth Infection in Ageing Mice
Abstract
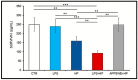
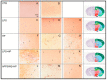
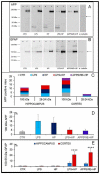
Parasitic helminths induce a transient, short-term inflammation at the beginning of infection, but in persistent infection may suppress the systemic immune response by enhancing the activity of regulatory M2 macrophages. The aim of the study was to determine how nematode infection affects age-related neuroinflammation, especially macrophages in the nervous tissue. Here, intraperitoneal LPS-induced systemic inflammation resulting in brain neurodegeneration was enhanced by prolonged Heligmosomoides polygyrus infection in C57BL/6 mice. The changes in the brain coincided with the increase in M1 macrophages, reduced survivin level, enhanced APP and GFAP expression, chitin-like chains deposition in the brain and deterioration behaviour manifestations. These changes were also observed in transgenic C57BL/6 mice predisposed to develop neurodegeneration typical for Alzheimer's disease in response to pathogenic stimuli. Interestingly, in mice infected with the nematode only, the greater M2 macrophage population resulted in better results in the forced swim test. Given the growing burden of neurodegenerative diseases, understanding such interactive associations can have significant implications for ageing health strategies and disease monitoring.
Keywords: ageing; helminths; immunomodulation; neurobehavioural manifestations; neurodegenerative diseases; neuroinflammation; persistent infection.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Battaglia S., Di Fazio C., Vicario C.M., Avenanti A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023;24:5926. doi: 10.3390/ijms24065926. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

