Reduced Sphingosine in Cystic Fibrosis Increases Susceptibility to Mycobacterium abscessus Infections
- PMID: 37762308
- PMCID: PMC10530875
- DOI: 10.3390/ijms241814004
Reduced Sphingosine in Cystic Fibrosis Increases Susceptibility to Mycobacterium abscessus Infections
Abstract
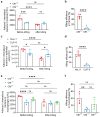
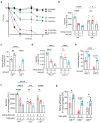
Cystic fibrosis (CF) is an autosomal recessive disorder caused by the deficiency of the cystic fibrosis transmembrane conductance regulator (CFTR) and often leads to pulmonary infections caused by various pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, and nontuberculous mycobacteria, particularly Mycobacterium abscessus. Unfortunately, M. abscessus infections are increasing in prevalence and are associated with the rapid deterioration of CF patients. The treatment options for M. abscessus infections are limited, requiring the urgent need to comprehend infectious pathogenesis and develop new therapeutic interventions targeting affected CF patients. Here, we show that the deficiency of CFTR reduces sphingosine levels in bronchial and alveolar epithelial cells and macrophages from CF mice and humans. Decreased sphingosine contributes to the susceptibility of CF tissues to M. abscessus infection, resulting in a higher incidence of infections in CF mice. Notably, treatment of M. abscessus with sphingosine demonstrated potent bactericidal activity against the pathogen. Most importantly, restoration of sphingosine levels in CF cells, whether human or mouse, and in the lungs of CF mice, provided protection against M. abscessus infections. Our findings demonstrate that pulmonary sphingosine levels are important in controlling M. abscessus infection. These results offer a promising therapeutic avenue for CF patients with pulmonary M. abscessus infections.
Keywords: Mycobacterium abscessus; cystic fibrosis; sphingolipids; sphingosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Combined Host- and Pathogen-Directed Therapy for the Control of Mycobacterium abscessus Infection.Microbiol Spectr. 2022 Feb 23;10(1):e0254621. doi: 10.1128/spectrum.02546-21. Epub 2022 Jan 26. Microbiol Spectr. 2022. PMID: 35080463 Free PMC article.
-
Pulmonary nontuberculous mycobacterial infections among women with cystic fibrosis and non-cystic fibrosis bronchiectasis.Ther Adv Respir Dis. 2025 Jan-Dec;19:17534666251323181. doi: 10.1177/17534666251323181. Epub 2025 Mar 12. Ther Adv Respir Dis. 2025. PMID: 40071337 Free PMC article. Review.
-
Bacterial phospholipases C as vaccine candidate antigens against cystic fibrosis respiratory pathogens: the Mycobacterium abscessus model.Vaccine. 2015 Apr 27;33(18):2118-24. doi: 10.1016/j.vaccine.2015.03.030. Epub 2015 Mar 21. Vaccine. 2015. PMID: 25804706
-
IgA Serological Response for the Diagnosis of Mycobacterium abscessus Infections in Patients with Cystic Fibrosis.Microbiol Spectr. 2022 Jun 29;10(3):e0019222. doi: 10.1128/spectrum.00192-22. Epub 2022 May 18. Microbiol Spectr. 2022. PMID: 35583329 Free PMC article.
-
Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients.Int J Mol Sci. 2019 Nov 22;20(23):5868. doi: 10.3390/ijms20235868. Int J Mol Sci. 2019. PMID: 31766758 Free PMC article. Review.
Cited by
-
The Pseudomonas aeruginosa sphBC genes are important for growth in the presence of sphingosine by promoting sphingosine metabolism.bioRxiv [Preprint]. 2024 Sep 3:2024.09.03.611043. doi: 10.1101/2024.09.03.611043. bioRxiv. 2024. Update in: Microbiology (Reading). 2025 Jan;171(1). doi: 10.1099/mic.0.001520. PMID: 39282278 Free PMC article. Updated. Preprint.
-
Insights on the Pathogenesis of Mycobacterium abscessus Infection in Patients with Cystic Fibrosis.J Clin Med. 2025 May 16;14(10):3492. doi: 10.3390/jcm14103492. J Clin Med. 2025. PMID: 40429486 Free PMC article. Review.
-
Protein interactions, calcium, phosphorylation, and cholesterol modulate CFTR cluster formation on membranes.Proc Natl Acad Sci U S A. 2025 Mar 18;122(11):e2424470122. doi: 10.1073/pnas.2424470122. Epub 2025 Mar 10. Proc Natl Acad Sci U S A. 2025. PMID: 40063811 Free PMC article.
-
The Pseudomonas aeruginosa sphBC genes are important for growth in the presence of sphingosine by promoting sphingosine metabolism.Microbiology (Reading). 2025 Jan;171(1):001520. doi: 10.1099/mic.0.001520. Microbiology (Reading). 2025. PMID: 39791474 Free PMC article.
-
Sphingosine kills Mycobacteria and suppresses mycobacterial lung infections.J Mol Med (Berl). 2025 May;103(5):547-558. doi: 10.1007/s00109-025-02534-z. Epub 2025 Mar 28. J Mol Med (Berl). 2025. PMID: 40153002 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

