miR-29a Is Downregulated in Progenies Derived from Chronically Stressed Males
- PMID: 37762407
- PMCID: PMC10531283
- DOI: 10.3390/ijms241814107
miR-29a Is Downregulated in Progenies Derived from Chronically Stressed Males
Abstract
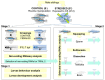
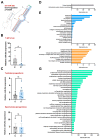
Recent research has provided compelling evidence demonstrating that paternal exposure to different stressors can influence their offspring's phenotypes. We hypothesized that paternal stress can negatively impact the progeny, altering different miRs and triggering different physiological alterations that could compromise offspring development. To investigate this, we exposed zebrafish male siblings to a chronic stress protocol for 21 days. We performed RNA-sequencing (RNA-seq) analyses to identify differentially expressed small noncoding RNAs in 7-day postfertilization (dpf) larvae derived from paternally stressed males crossed with control females compared with the control progeny. We found a single miRNA differentially expressed-miR-29a-which was validated in larva and was also tested in the sperm, testicles, and brain of the stressed progenitors. We observed a vertical transmission of chronic stress to the unexposed larvae, reporting novel consequences of paternally inherited chronic stress at a molecular level. The deregulation of mi-R29a in those larvae could affect relevant biological processes affecting development, morphogenesis, or neurogenesis, among others. Additionally, these disruptions were associated with reduced rates of survival and hatching in the affected offspring.
Keywords: RNA-seq; behavior; chronic stress; hatching rates; malformations; offspring; small-RNAs; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schreck C.B., Tort L. The Concept of Stress in Fish. Fish Physiol. 2016;35:1–34. doi: 10.1016/B978-0-12-802728-8.00001-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

