Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation
- PMID: 37762414
- PMCID: PMC10531309
- DOI: 10.3390/ijms241814112
Time-Dependent Changes in Muscle IGF1-IGFBP5-PAPP System after Sciatic Denervation
Abstract
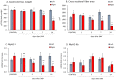
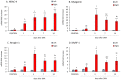
Denervation-induced muscle atrophy is a frequent cause of skeletal muscle diseases. However, the role of the most important muscle growth factor, insulin-like growth factor (IGF-1), in this process is poorly understood. IGF-1 activity is controlled by six IGF-1 binding proteins (IGFBPs). In skeletal muscle, IGFBP-5 seems to have an important role in atrophic processes. Furthermore, pappalysins (PAPP-A) modulate muscle growth by increasing IGF-1 bioavailability through IGFBP cleavage. We aimed to study the time-dependent changes in the IGF1-IGFBP5-PAPP system and its regulators in gastrocnemius muscle after sciatic denervation. Gastrocnemius atrophy and overexpression of IGF-1 was observed from day 3 post-denervation. The proteolytic factors measured were elevated from day 1 post-denervation onwards. Expression of both IGFBP-5 and pappalysins were increased on days 1 and 3. Subsequently, on days 7 to 14 pappalysins returned to control levels while IGFBP-5 remained elevated. The ratio IGFBP-5/PAPP-A was correlated with the main proteolytic markers. All data suggest that the initial increase of pappalysins could facilitate the IGF-1 action on muscle growth, whereas their subsequent decrease could lead to further muscle wasting.
Keywords: IGF-1; IGFBP-5; pappalysins; skeletal muscle atrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

