The Biocompatibility Analysis of Artificial Mucin-Like Glycopolymers
- PMID: 37762451
- PMCID: PMC10532372
- DOI: 10.3390/ijms241814150
The Biocompatibility Analysis of Artificial Mucin-Like Glycopolymers
Abstract
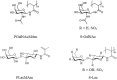
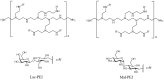
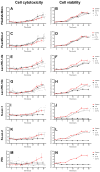
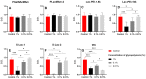
The ocular surface is covered by a tear film consisting of an aqueous/mucin phase and a superficial lipid layer. Mucins, highly O-glycosylated proteins, are responsible for lubrication and ocular surface protection. Due to contact lens wear or eye disorders, lubrication of the ocular surface can be affected. Artificial glycopolymers which mimic natural mucins could be efficient in ophthalmic therapy. Various neutral, positively, and negatively charged mucin-mimicking glycopolymers were synthesized (n = 11), cultured in different concentrations (1%, 0.1%, and 0.01% w/v) with human corneal epithelial cells (HCE), and analyzed by various cytotoxicity/viability, morphology, and immunohistochemistry (IHC) assays. Six of the eleven glycopolymers were selected for further analysis after cytotoxicity/viability assays. We showed that the six selected glycopolymers had no cytotoxic effect on HCE cells in the 0.01% w/v concentration. They did not negatively affect cell viability and displayed both morphology and characteristic markers as untreated control cells. These polymers could be used in the future as mucin-mimicking semi-synthetic materials for lubrication and protection of the ocular surface.
Keywords: cornea; epithelial corneal cells; glycopolymers; mucin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Foss A.J., Trodd T.C., Dart J.K. Current indications for scleral contact lenses. CLAO J. 1994;20:115–118. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

