New Insights in Immunometabolism in Neonatal Monocytes and Macrophages in Health and Disease
- PMID: 37762476
- PMCID: PMC10531550
- DOI: 10.3390/ijms241814173
New Insights in Immunometabolism in Neonatal Monocytes and Macrophages in Health and Disease
Abstract
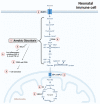
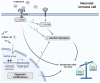
It is well established that the neonatal immune system is different from the adult immune system. A major task of the neonatal immune system is to bridge the achievement of tolerance towards harmless antigens and commensal bacteria while providing protection against pathogens. This is highly important because neonates are immunologically challenged directly after birth by a rigorous change from a semi-allogeneic sterile environment into a world rich with microbes. A so called disease tolerogenic state is typical for neonates and is anticipated to prevent immunopathological damage potentially at the cost of uncontrolled pathogen proliferation. As a consequence, neonates are more susceptible than adults to life-threatening infections. At the basis of a well-functioning immune response, both for adults and neonates, innate immune cells such as monocytes and monocyte-derived macrophages play an essential role. A well-responsive monocyte will alter its cellular metabolism to subsequently induce certain immune effector function, a process which is called immunometabolism. Immunometabolism has received extensive attention in the last decade; however, it has not been broadly studied in neonates. This review focuses on carbohydrate metabolism in monocytes and macrophages in neonates. We will exhibit pathways involving glycolysis, the tricarboxylic acid (TCA) cycle and oxidative phosphorylation and their role in shaping neonates' immune systems to a favorable tolerogenic state. More insight into these pathways will elucidate potential treatments targets in life-threatening conditions including neonatal sepsis or expose potential targets which can be used to induce tolerance in conditions where tolerance is harmfully impaired such as in autoimmune diseases.
Keywords: bacterial; commensals; neonatal infection; neonatal tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

