A High-Throughput Small-Angle X-ray Scattering Assay to Determine the Conformational Change of Plasminogen
- PMID: 37762561
- PMCID: PMC10531915
- DOI: 10.3390/ijms241814258
A High-Throughput Small-Angle X-ray Scattering Assay to Determine the Conformational Change of Plasminogen
Abstract
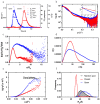
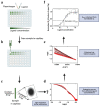
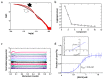
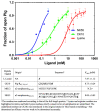
Plasminogen (Plg) is the inactive form of plasmin (Plm) that exists in two major glycoforms, referred to as glycoforms I and II (GI and GII). In the circulation, Plg assumes an activation-resistant "closed" conformation via interdomain interactions and is mediated by the lysine binding site (LBS) on the kringle (KR) domains. These inter-domain interactions can be readily disrupted when Plg binds to lysine/arginine residues on protein targets or free L-lysine and analogues. This causes Plg to convert into an "open" form, which is crucial for activation by host activators. In this study, we investigated how various ligands affect the kinetics of Plg conformational change using small-angle X-ray scattering (SAXS). We began by examining the open and closed conformations of Plg using size-exclusion chromatography (SEC) coupled with SAXS. Next, we developed a high-throughput (HTP) 96-well SAXS assay to study the conformational change of Plg. This method enables us to determine the Kopen value, which is used to directly compare the effect of different ligands on Plg conformation. Based on our analysis using Plg GII, we have found that the Kopen of ε-aminocaproic acid (EACA) is approximately three times greater than that of tranexamic acid (TXA), which is widely recognized as a highly effective ligand. We demonstrated further that Plg undergoes a conformational change when it binds to the C-terminal peptides of the inhibitor α2-antiplasmin (α2AP) and receptor Plg-RKT. Our findings suggest that in addition to the C-terminal lysine, internal lysine(s) are also necessary for the formation of open Plg. Finally, we compared the conformational changes of Plg GI and GII directly and found that the closed form of GI, which has an N-linked glycosylation, is less stable. To summarize, we have successfully determined the response of Plg to various ligand/receptor peptides by directly measuring the kinetics of its conformational changes.
Keywords: SAXS; conformational change; fibrinolysis; kringle domain; lysine analogue; lysine binding site; plasminogen; structure-function.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article.
Figures
References
-
- Castellino F.J., Ploplis V.A. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 2005;93:647–654. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

