Uridine as a Regulator of Functional and Ultrastructural Changes in the Brain of Rats in a Model of 6-OHDA-Induced Parkinson's Disease
- PMID: 37762607
- PMCID: PMC10531918
- DOI: 10.3390/ijms241814304
Uridine as a Regulator of Functional and Ultrastructural Changes in the Brain of Rats in a Model of 6-OHDA-Induced Parkinson's Disease
Abstract
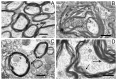
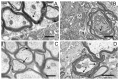
Using a model of Parkinson's disease (PD) induced by the bilateral injection of neurotoxin 6-hydroxydopamine (6-OHDA) into rat brain substantia nigra (SN), we showed uridine to exert a protective effect associated with activation of the mitochondrial ATP-dependent potassium (mitoK-ATP) channel. Injection of 4 µg neurotoxin evoked a 70% decrease in the time the experimental animal spent on the rod in the RotaRod test, an increase in the amount of lipid peroxides in blood serum and cerebral-cortex mitochondria and the rate of reactive oxygen species formation, and a decrease in Ca2+ retention in mitochondria. Herewith, lymphocytes featured an increase in the activity of lactate dehydrogenase, a cytosolic enzyme of glycolysis, without changes in succinate-dehydrogenase activity. Structural changes occurring in the SN and striatum manifested themselves in the destruction of mitochondria, degeneration of neurons and synapses, and stratification of myelin sheaths in them. Subcutaneous injections of 30 µg/kg uridine for 22 days restored the neurotoxin-induced changes in these parameters to levels close to the control. 5-Hydroxydecanoate (5 mg/kg), a specific mitoK-ATP channel inhibitor, eliminated the beneficial effect of uridine for almost all characteristics tested, indicating the involvement of the mitoK-ATP channel in the protective effect of uridine. The mechanism of the protective effect of uridine and its therapeutic applications for the prevention and treatment of PD are discussed.
Keywords: 6-hydroxydopamine; Parkinson’s disease; mitochondria; mitochondrial ATP-dependent potassium channel; neurons; oxidative stress; structure; synapses; uridine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lee T.K., Yankee E.L. A review on Parkinson’s disease treatment. Neuroimmunol. Neuroinflamm. 2021;8:222–244. doi: 10.20517/2347-8659.2020.58. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

