A Side-by-Side Comparison of Wildtype and Variant Melanocortin 1 Receptor Signaling with Emphasis on Protection against Oxidative Damage to DNA
- PMID: 37762683
- PMCID: PMC10532403
- DOI: 10.3390/ijms241814381
A Side-by-Side Comparison of Wildtype and Variant Melanocortin 1 Receptor Signaling with Emphasis on Protection against Oxidative Damage to DNA
Abstract
Common variants of the MC1R gene coding the α-melanocyte stimulating hormone receptor are associated with light skin, poor tanning, blond or red hair, and increased melanoma risk, due to pigment-dependent and -independent effects. This complex phenotype is usually attributed to impaired activation of cAMP signaling. However, several MC1R variants show significant residual coupling to cAMP and efficiently activate mitogenic extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling. Yet, residual signaling and the key actions of wildtype and variant MC1R have never been assessed under strictly comparable conditions in melanocytic cells of identical genetic background. We devised a strategy based on CRISPR-Cas9 knockout of endogenous MC1R in a human melanoma cell line wildtype for BRAF, NRAS and NF1, followed by reconstitution with epitope-labeled MC1R constructs, and functional analysis of clones expressing comparable levels of wildtype, R151C or D294H MC1R. The proliferation rate, shape, adhesion, motility and sensitivity to oxidative DNA damage were compared. The R151C and D294H RHC variants displayed impaired cAMP signaling, intracellular stability similar to the wildtype, triggered ERK1/2 activation as effectively as the wildtype, and afforded partial protection against oxidative DNA damage, although less efficiently than the wildtype. Therefore, common melanoma-associated MC1R variants display biased signaling and significant genoprotective activity.
Keywords: DNA damage; cAMP; extracellular signal-regulated kinases 1 and 2 (ERK1/2); melanocortin 1 receptor (MC1R); melanoma; proliferation; signaling; variant MC1R.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

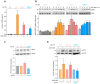

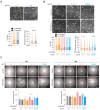
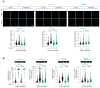
References
-
- Tagliabue E., Gandini S., Bellocco R., Maisonneuve P., Newton-Bishop J., Polsky D., Lazovich D., Kanetsky P.A., Ghiorzo P., Gruis N.A., et al. MC1R Variants as Melanoma Risk Factors Independent of At-Risk Phenotypic Characteristics: A Pooled Analysis from the M-SKIP Project. Cancer Manag. Res. 2018;10:1143–1154. doi: 10.2147/CMAR.S155283. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

