LOXL2 in Cancer: A Two-Decade Perspective
- PMID: 37762708
- PMCID: PMC10532419
- DOI: 10.3390/ijms241814405
LOXL2 in Cancer: A Two-Decade Perspective
Abstract
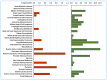
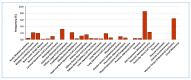
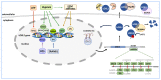
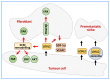
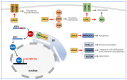
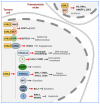
Lysyl Oxidase Like 2 (LOXL2) belongs to the lysyl oxidase (LOX) family, which comprises five lysine tyrosylquinone (LTQ)-dependent copper amine oxidases in humans. In 2003, LOXL2 was first identified as a promoter of tumour progression and, over the course of two decades, numerous studies have firmly established its involvement in multiple cancers. Extensive research with large cohorts of human tumour samples has demonstrated that dysregulated LOXL2 expression is strongly associated with poor prognosis in patients. Moreover, investigations have revealed the association of LOXL2 with various targets affecting diverse aspects of tumour progression. Additionally, the discovery of a complex network of signalling factors acting at the transcriptional, post-transcriptional, and post-translational levels has provided insights into the mechanisms underlying the aberrant expression of LOXL2 in tumours. Furthermore, the development of genetically modified mouse models with silenced or overexpressed LOXL2 has enabled in-depth exploration of its in vivo role in various cancer models. Given the significant role of LOXL2 in numerous cancers, extensive efforts are underway to identify specific inhibitors that could potentially improve patient prognosis. In this review, we aim to provide a comprehensive overview of two decades of research on the role of LOXL2 in cancer.
Keywords: LOXL2; human tumour sample; mouse models; regulation; targets; tumour progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

