Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation
- PMID: 37762726
- PMCID: PMC10532406
- DOI: 10.3390/jcm12185784
Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation
Abstract
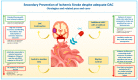
Patients with atrial fibrillation (AF) experiencing ischemic stroke despite oral anticoagulation (OAC), i.e., breakthrough strokes, are not uncommon, and represent an important clinical subgroup in view of the consistently high risk of stroke recurrence and mortality. The understanding of the heterogenous potential mechanism underlying OAC failure is essential in order to implement specific therapeutic measures aimed at reducing the risk of recurrent ischemic stroke. However, due to the incomplete comprehension of this phenomenon and the limited available data, secondary stroke prevention in such high-risk patients represents a clinical dilemma. There are several available strategies to prevent ischemic stroke recurrence in AF patients with breakthrough stroke in the absence of competing causes unrelated to AF, and these include continuation or change in the type of OAC, addition of antiplatelet therapy, left atrial appendage closure, or any combination of the above options. However, due to the limited available data, the latest guidelines do not provide any specific recommendations about which of the above strategies may be preferred. This review describes the incidence, the clinical impact and the potential mechanisms underlying OAC failure in AF patients. Furthermore, the evidence supporting each of the above therapeutic options for secondary stroke prevention and the potential future directions will be discussed.
Keywords: OAC failure; breakthrough strokes; ischemic stroke; left atrial appendage closure; stroke under OAC.
Conflict of interest statement
LR reports research grants to the institution by Abbott-Vascular, Boston-Scientific, Biotronik, Infraredx, Heartflow, Sanofi, the Swiss National Science Foundation, the Swiss Heart Foundation, and Regeneron. He reports speaker/consultation fees from Abbott-Vascular, Amgen, AstraZeneca, CSL-Behring, Canon, Occlutech, Sanofi, and Vifor. DJS reports research grants to the institution from the Swiss National Science Foundation, the Swiss Heart Foundation, the Bangerter-Rhyner Foundation, and Alexion/AstraZeneca. Personal fees to the institution were from Bayer, VarmX, AstraZeneca, and Pfizer. All other authors have reported that they have no relationships relevant to the content of this paper to disclose.
Figures
References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. - DOI - PubMed
-
- Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.H., McAnulty J.H., Jr., Zheng Z.J., et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. - DOI - PMC - PubMed
-
- Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomstrom-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

