First-in-Human Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques: Rationale and Design of the DEBuT-LRP Study
- PMID: 37762747
- PMCID: PMC10531515
- DOI: 10.3390/jcm12185807
First-in-Human Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques: Rationale and Design of the DEBuT-LRP Study
Erratum in
-
Correction: van Veelen et al. First-in-Human Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques: Rationale and Design of the DEBuT-LRP Study. J. Clin. Med. 2023, 12, 5807.J Clin Med. 2024 Mar 4;13(5):1479. doi: 10.3390/jcm13051479. J Clin Med. 2024. PMID: 38592321 Free PMC article.
Abstract
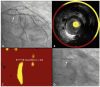
Patients with non-obstructive lipid-rich plaques (LRPs) on combined intravascular ultrasound (IVUS) and near-infrared spectroscopy (NIRS) are at high risk for future events. Local pre-emptive percutaneous treatment of LRPs with a paclitaxel-eluting drug-coated balloon (PE-DCB) may be a novel therapeutic strategy to prevent future adverse coronary events without leaving behind permanent coronary implants. In this pilot study, we aim to investigate the safety and feasibility of pre-emptive treatment with a PE-DCB of non-culprit non-obstructive LRPs by evaluating the change in maximum lipid core burden in a 4 mm segment (maxLCBImm4) after 9 months of follow up. Therefore, patients with non-ST-segment elevation acute coronary syndrome underwent 3-vessel IVUS-NIRS after treatment of the culprit lesion to identify additional non-obstructive non-culprit LRPs, which were subsequently treated with PE-DCB sized 1:1 to the lumen. We enrolled 45 patients of whom 20 patients (44%) with a non-culprit LRP were treated with PE-DCB. After 9 months, repeat coronary angiography with IVUS-NIRS will be performed. The primary endpoint at 9 months is the change in maxLCBImm4 in PE-DCB-treated LRPs. Secondary endpoints include clinical adverse events and IVUS-derived parameters such as plaque burden and luminal area. Clinical follow-up will continue until 1 year after enrollment. In conclusion, this first-in-human study will investigate the safety and feasibility of targeted pre-emptive PE-DCB treatment of LRPs to promote stabilization of vulnerable coronary plaque at risk for developing future adverse events.
Keywords: drug-coated balloon; intracoronary imaging; intravascular ultrasound; near-infrared spectroscopy; non-ST-segment elevation acute coronary syndrome; vulnerable plaque.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Waksman R., Di Mario C., Torguson R., Ali Z.A., Singh V., Skinner W.H., Artis A.K., Cate T.T., Powers E., Kim C., et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet. 2019;394:1629–1637. doi: 10.1016/S0140-6736(19)31794-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

