Harnessing Immune Response in Acute Myeloid Leukemia
- PMID: 37762763
- PMCID: PMC10532363
- DOI: 10.3390/jcm12185824
Harnessing Immune Response in Acute Myeloid Leukemia
Abstract
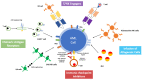
Despite the results achieved with the evolution of conventional chemotherapy and the inclusion of targeted therapies in the treatment of acute myeloid leukemia (AML), survival is still not satisfying, in particular in the setting of relapsed/refractory (R/R) disease or elderly/unfit patients. Among the most innovative therapeutic options, cellular therapy has shown great results in different hematological malignancies such as acute lymphoblastic leukemia and lymphomas, with several products already approved for clinical use. However, despite the great interest in also expanding the application of these new treatments to R/R AML, no product has been approved yet for clinical application. Furthermore, cellular therapy could indeed represent a powerful tool and an appealing alternative to allogeneic hematopoietic stem cell transplantation for ineligible patients. In this review, we aim to provide an overview of the most recent clinical research exploring the effectiveness of cellular therapy in AML, moving from consolidated approaches such as post- transplant donor's lymphocytes infusion, to modern adoptive immunotherapies such as alloreactive NK cell infusions, engineered T and NK cells (CAR-T, CAR-NK) and novel platforms of T and NK cells engaging (i.e., BiTEs, DARTs and ANKETTM).
Keywords: AML; CAR-T; NK cells; immunotherapy.
Conflict of interest statement
The authors state that they have no relevant conflict of interest to disclose.
Figures

References
-
- Döhner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

