Role of Crystalloids in the Perioperative Setting: From Basics to Clinical Applications and Enhanced Recovery Protocols
- PMID: 37762871
- PMCID: PMC10531658
- DOI: 10.3390/jcm12185930
Role of Crystalloids in the Perioperative Setting: From Basics to Clinical Applications and Enhanced Recovery Protocols
Abstract
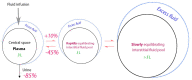
Perioperative fluid management, a critical aspect of major surgeries, is characterized by pronounced stress responses, altered capillary permeability, and significant fluid shifts. Recognized as a cornerstone of enhanced recovery protocols, effective perioperative fluid management is crucial for optimizing patient recovery and preventing postoperative complications, especially in high-risk patients. The scientific literature has extensively investigated various fluid infusion regimens, but recent publications indicate that not only the volume but also the type of fluid infused significantly influences surgical outcomes. Adequate fluid therapy prescription requires a thorough understanding of the physiological and biochemical principles that govern the body's internal environment and the potential perioperative alterations that may arise. Recently published clinical trials have questioned the safety of synthetic colloids, widely used in the surgical field. A new clinical scenario has arisen in which crystalloids could play a pivotal role in perioperative fluid therapy. This review aims to offer evidence-based clinical principles for prescribing fluid therapy tailored to the patient's physiology during the perioperative period. The approach combines these principles with current recommendations for enhanced recovery programs for surgical patients, grounded in physiological and biochemical principles.
Keywords: enhanced recovery after surgery; fluid therapy; postoperative complications.
Conflict of interest statement
J.V.L. (Juan V. Lorente): Payment for courses, research, and conferences from Edwards Lifesciences and bioMérieux. Payment for courses and conferences from Fresenius Kabi, Grifols, Vifor Pharma and Baxter. R.G.H.: The author declares no conflict of interest related to the article. J.L.J.: Honoraria for Baxter and Fresenius Kabi conferences. E.D.C.: Conference fees and clinical consultancy for Teleflex, MBA, BBRAUN, and Grünenthal Pharma. M.H.: The author declares no conflict of interest related to the article. I.J.: Payment for courses and conferences from Edwards Lifesciences. R.U.: Honoraria for Baxter conferences. F.C.-T.: The author declares no conflict of interest related to the article. M.I.M.: Payment for conferences, courses, and research from Edwards Lifesciences; acted as medical advisor for Edwards Lifesciences. J.V.L. (Juan V. Llau): The author declares no conflict of interest related to the article. M.J.C.: Payments for conferences from Octapharma, C.S.L. Behring, and Fresenius Kabi. J.R.-M.: Payments for conferences, courses, and research from Edwards Lifesciences; payments for conferences from Biomerieux and Fresenius Kabi.
Figures


References
-
- Navarro L.H.C., Bloomstone J.A., Auler J.O.C., Cannesson M., Rocca G.D., Gan T.J., Kinsky M., Magder S., Miller T.E., Mythen M., et al. Perioperative Fluid Therapy: A Statement from the International Fluid Optimization Group. Perioper. Med. 2015;4:3. doi: 10.1186/s13741-015-0014-z. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

