Eosinophilic Granulomatosis with Polyangiitis: Latest Findings and Updated Treatment Recommendations
- PMID: 37762936
- PMCID: PMC10532073
- DOI: 10.3390/jcm12185996
Eosinophilic Granulomatosis with Polyangiitis: Latest Findings and Updated Treatment Recommendations
Abstract
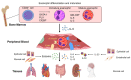
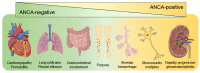
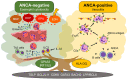
Eosinophilic granulomatosis with polyangiitis (EGPA) causes necrotizing vasculitis and eosinophil-rich granulomatous inflammation in small- to medium-sized vessels, resulting in multiple organ damage. EGPA is classified as an antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, with myeloperoxidase-ANCA detected in approximately one-third of the patients. Conventional treatment of EGPA relies on systemic glucocorticoids (GCs) in combination with cyclophosphamide when poor prognostic factors are present; however, the dilemma between disease control and drug-related adverse effects has long been a challenge. Recent studies have revealed that the genetic background, pathophysiology, and clinical manifestations differ between ANCA-positive and ANCA-negative patients; however, mepolizumab, an interleukin (IL)-5 inhibitor, is effective in both groups, suggesting that the IL-5-eosinophil axis is deeply involved in the pathogenesis of both ANCA-positive and ANCA-negative EGPA. This review summarizes the latest knowledge on the pathophysiology of EGPA and focuses on the roles of eosinophils and ANCA. We then introduce the current treatment recommendations and accumulated evidence for mepolizumab on EGPA. Based on current unmet clinical needs, we discuss potential future therapeutic strategies for EGPA.
Keywords: antineutrophil cytoplasmic antibody; eosinophil; eosinophilic granulomatosis with polyangiitis; interleukin-5; mepolizumab.
Conflict of interest statement
R.W. received a research grant from AbbVie and speaker fee from Asahi Kasei, Chugai, Eli Lilly, and GSK. M.H. received research grants and/or speaker fee from AbbVie, Asahi Kasei, Astellas, Bristol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, Taisho Toyama, or Tanabe Mitsubishi. These pharmaceutical companies had no role in the writing of the manuscript.
Figures

References
-
- Valent P., Klion A.D., Roufosse F., Simon D., Metzgeroth G., Leiferman K.M., Schwaab J., Butterfield J.H., Sperr W.R., Sotlar K., et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2023;78:47–59. doi: 10.1111/all.15544. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

