Nanoparticle-Based Treatment in Glioblastoma
- PMID: 37763096
- PMCID: PMC10532799
- DOI: 10.3390/jpm13091328
Nanoparticle-Based Treatment in Glioblastoma
Abstract
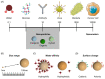
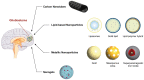
Glioblastoma (GB) is a malignant glioma associated with a mean overall survival of 12 to 18 months, even with optimal treatment, due to its high relapse rate and treatment resistance. The standardized first-line treatment consists of surgery, which allows for diagnosis and cytoreduction, followed by stereotactic fractionated radiotherapy and chemotherapy. Treatment failure can result from the poor passage of drugs through the blood-brain barrier (BBB). The development of novel and more effective therapeutic approaches is paramount to increasing the life expectancy of GB patients. Nanoparticle-based treatments include epitopes that are designed to interact with specialized transport systems, ultimately allowing the crossing of the BBB, increasing therapeutic efficacy, and reducing systemic toxicity and drug degradation. Polymeric nanoparticles have shown promising results in terms of precisely directing drugs to the brain with minimal systemic side effects. Various methods of drug delivery that pass through the BBB, such as the stereotactic injection of nanoparticles, are being actively tested in vitro and in vivo in animal models. A significant variety of pre-clinical studies with polymeric nanoparticles for the treatment of GB are being conducted, with only a few nanoparticle-based drug delivery systems to date having entered clinical trials. Pre-clinical studies are key to testing the safety and efficacy of these novel anticancer therapies and will hopefully facilitate the testing of the clinical validity of this promising treatment method. Here we review the recent literature concerning the most frequently reported types of nanoparticles for the treatment of GB.
Keywords: blood-brain barrier; glioblastoma; nanoparticles; therapy delivery.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

