Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation
- PMID: 37763216
- PMCID: PMC10532860
- DOI: 10.3390/life13091812
Adiponectin Modulates Smooth Muscle Cell Morpho-Functional Properties in Murine Gastric Fundus via Sphingosine Kinase 2 Activation
Abstract
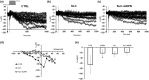
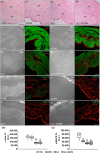
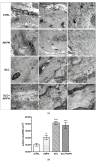
Adipokines are peptide hormones produced by the adipose tissue involved in several biological functions. Among adipokines, adiponectin (ADPN) has antidiabetic and anti-inflammatory properties. It can also modulate food intake at central and peripheral levels, acting on hypothalamus and facilitating gastric relaxation. ADPN exerts its action interacting with two distinct membrane receptors and triggering some well-defined signaling cascades. The ceramidase activity of ADPN receptor has been reported in many tissues: it converts ceramide into sphingosine. In turn, sphingosine kinase (SK) phosphorylates it into sphingosine-1 phosphate (S1P), a crucial mediator of many cellular processes including contractility. Using a multidisciplinary approach that combined biochemical, electrophysiological and morphological investigations, we explored for the first time the possible role of S1P metabolism in mediating ADPN effects on the murine gastric fundus muscle layer. By using a specific pharmacological inhibitor of SK2, we showed that ADPN affects smooth muscle cell membrane properties and contractile machinery via SK2 activation in gastric fundus, adding a piece of knowledge to the action mechanisms of this hormone. These findings help to identify ADPN and its receptors as new therapeutic targets or as possible prognostic markers for diseases with altered energy balance and for pathologies with fat mass content alterations.
Keywords: adiponectin; gastric fundus; membrane properties; morphology; signaling pathway; smooth muscle; sphingosine kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


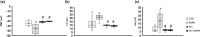

References
Grants and funding
LinkOut - more resources
Full Text Sources

