Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression
- PMID: 37763221
- PMCID: PMC10532713
- DOI: 10.3390/life13091817
Total and Extracellular Vesicle cAMP Contents in Urine Are Associated with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Progression
Abstract
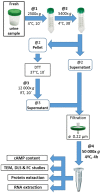
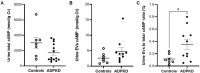
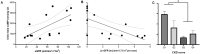
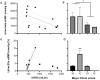
ADPKD is the most common genetic renal disease, characterized by the presence of multiple cysts which, through slow and gradual growth, lead to glomerular filtration rate (GFR) decline and end-stage renal disease. Cystic growth is associated with increased intracellular levels of 3',5'-cyclic adenosine monophosphate (cAMP). Extracellular vesicles (EVs) are proposed to participate in "remote sensing" by transporting different cargoes, but their relevance to ADPKD progression is poorly understood. This study aimed to determine whether cAMP is contained in urinary EVs and, if so, how total and/or EV cAMP contents participate in disease progression. Fourteen ADPKD patients, naïve for V2 receptor antagonism treatment, and seven controls were studied. Progression was evaluated by estimating GFR (eGFR) and height-adjusted total kidney volume (htTKV). Fresh morning urine was collected to determine cAMP by the competitive radioligand assay. Urine EVs were isolated using an adapted centrifugation method and characterized by electron microscopy, dynamic light scanning, flow cytometry with FITC CD63 labeling, protein and RNA content, and AQP2 and GAPDH mRNA detection. Total and EV cAMP was measurable in both control and patient urine samples. Total cAMP was significantly correlated with eGFR and its annual change but inversely correlated with htTKV. The cAMP-EVs showed a bimodal pattern with htTKV, increasing to ~1 L/m and falling at larger sizes. Our results demonstrate that urine cAMP correlates with ADPKD progression markers, and that its extracellular delivery by EVs could reflect the architectural disturbances of the organ.
Keywords: ADPKD progression; cyclic AMP; cystic growth; urine extracellular vesicles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
Figures


References
-
- Tazón-Vega B., Vilardell M., Pérez-Oller L., Ars E., Ruiz P., Devuyst O., Lens X., Fernández-Llama P., Ballarín J., Torra R. Study of candidate genes affecting the progression of renal disease in autosomal dominant polycystic kidney disease type 1. Nephrol. Dial. Transplant. 2007;22:1567–1577. doi: 10.1093/ndt/gfm036. - DOI - PubMed
-
- Ferreira F.M., Watanabe E.H., Onuchic L.F. Polycystins and Molecular Basis of Autosomal Dominant Polycystic Kidney Disease. In: Li X., editor. Polycystic Kidney Disease. Codon Publications; Brisbane, Australia: 2015. pp. 139–167. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

