Microscale Electrochemical Corrosion of Uranium Oxide Particles
- PMID: 37763890
- PMCID: PMC10537459
- DOI: 10.3390/mi14091727
Microscale Electrochemical Corrosion of Uranium Oxide Particles
Abstract
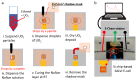
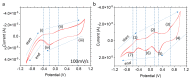
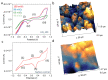
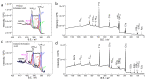
Understanding the corrosion of spent nuclear fuel is important for the development of long-term storage solutions. However, the risk of radiation contamination presents challenges for experimental analysis. Adapted from the system for analysis at the liquid-vacuum interface (SALVI), we developed a miniaturized uranium oxide (UO2)-attached working electrode (WE) to reduce contamination risk. To protect UO2 particles in a miniatured electrochemical cell, a thin layer of Nafion was formed on the surface. Atomic force microscopy (AFM) shows a dense layer of UO2 particles and indicates their participation in electrochemical reactions. Particles remain intact on the electrode surface with slight redistribution. X-ray photoelectron spectroscopy (XPS) reveals a difference in the distribution of U(IV), U(V), and U(VI) between pristine and corroded UO2 electrodes. The presence of U(V)/U(VI) on the corroded electrode surface demonstrates that electrochemically driven UO2 oxidation can be studied using these cells. Our observations of U(V) in the micro-electrode due to the selective semi-permeability of Nafion suggest that interfacial water plays a key role, potentially simulating a water-lean scenario in fuel storage conditions. This novel approach offers analytical reproducibility, design flexibility, a small footprint, and a low irradiation dose, while separating the α-effect. This approach provides a valuable microscale electrochemical platform for spent fuel corrosion studies with minimal radiological materials and the potential for diverse configurations.
Keywords: Nafion membrane; microscale electrochemical cell; multimodal characterization; particle-attached electrode; system for analysis at the liquid–vacuum interface (SALVI); uranium oxide (UO2).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Bruno J., Duro L., Diaz-Maurin F. Advances in Nuclear Fuel Chemistry. Volume 13. Woodhead Publishing; Sawston, UK: 2020. Chapter 13—Spent nuclear fuel and disposal; pp. 527–553.
-
- Standish T., Chen J., Jacklin R., Jakupi P., Ramamurthy S., Zagidulin D., Keech P., Shoesmith D. Corrosion of copper-coated steel high level nuclear waste containers under permanent disposal conditions. Electrochim. Acta. 2016;211:331–342. doi: 10.1016/j.electacta.2016.05.135. - DOI
-
- Shoesmith D.W. Fuel corrosion processes under waste disposal conditions. J. Nucl. Mater. 2000;282:1–31. doi: 10.1016/S0022-3115(00)00392-5. - DOI
-
- Kumari I., Kumar B.V.R., Khanna A. A review on urex processes for nuclear spent fuel reprocessing. Nucl. Eng. 2020;358:110410. doi: 10.1016/j.nucengdes.2019.110410. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

