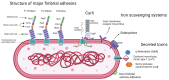
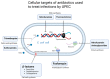
Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment
- PMID: 37764013
- PMCID: PMC10537683
- DOI: 10.3390/microorganisms11092169
Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment
Abstract
Urinary tract infections (UTIs) are among the most common bacterial infections, especially among women and older adults, leading to a significant global healthcare cost burden. Uropathogenic Escherichia coli (UPEC) are the most common cause and accounts for the majority of community-acquired UTIs. Infection by UPEC can cause discomfort, polyuria, and fever. More serious clinical consequences can result in urosepsis, kidney damage, and death. UPEC is a highly adaptive pathogen which presents significant treatment challenges rooted in a complex interplay of molecular factors that allow UPEC to evade host defences, persist within the urinary tract, and resist antibiotic therapy. This review discusses these factors, which include the key genes responsible for adhesion, toxin production, and iron acquisition. Additionally, it addresses antibiotic resistance mechanisms, including chromosomal gene mutations, antibiotic deactivating enzymes, drug efflux, and the role of mobile genetic elements in their dissemination. Furthermore, we provide a forward-looking analysis of emerging alternative therapies, such as phage therapy, nano-formulations, and interventions based on nanomaterials, as well as vaccines and strategies for immunomodulation. This review underscores the continued need for research into the molecular basis of pathogenesis and antimicrobial resistance in the treatment of UPEC, as well as the need for clinically guided treatment of UTIs, particularly in light of the rapid spread of multidrug resistance.
Keywords: antibiotic resistance; biofilm formation; mobile genetic elements; uropathogenic E. coli.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Genome-Wide Discovery of Genes Required for Capsule Production by Uropathogenic Escherichia coli.mBio. 2017 Oct 24;8(5):e01558-17. doi: 10.1128/mBio.01558-17. mBio. 2017. PMID: 29066548 Free PMC article.
-
Phenotypic Assessment of Clinical Escherichia coli Isolates as an Indicator for Uropathogenic Potential.mSystems. 2022 Dec 20;7(6):e0082722. doi: 10.1128/msystems.00827-22. Epub 2022 Nov 29. mSystems. 2022. PMID: 36445110 Free PMC article.
-
Antibiotic Resistance Among Uropathogenic Escherichia coli.Pol J Microbiol. 2019 Dec;68(4):403-415. doi: 10.33073/pjm-2019-048. Epub 2019 Dec 5. Pol J Microbiol. 2019. PMID: 31880885 Free PMC article. Review.
-
Biofilm formation by uropathogenic Escherichia coli: a complicating factor for treatment and recurrence of urinary tract infections.J Hosp Infect. 2021 Nov;117:9-16. doi: 10.1016/j.jhin.2021.08.017. Epub 2021 Aug 21. J Hosp Infect. 2021. PMID: 34428502
-
Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options.Int J Mol Sci. 2023 Jun 23;24(13):10537. doi: 10.3390/ijms241310537. Int J Mol Sci. 2023. PMID: 37445714 Free PMC article. Review.
Cited by
-
Synergistic Antibacterial Effects of Plant Extracts and Essential Oils Against Drug-Resistant Bacteria of Clinical Interest.Pathogens. 2025 Apr 4;14(4):348. doi: 10.3390/pathogens14040348. Pathogens. 2025. PMID: 40333114 Free PMC article.
-
Harnessing intracellular bacteria in bladder by intravesical delivery of antibiotics-loaded nanodiamonds to reduce the recurrence of urinary tract infection.J Nanobiotechnology. 2025 May 21;23(1):367. doi: 10.1186/s12951-025-03459-y. J Nanobiotechnology. 2025. PMID: 40394627 Free PMC article.
-
Broad-Spectrum Antimicrobial and Antibiofilm Activities of Halogenated Anilines Against Uropathogenic Escherichia coli and ESKAPE Pathogens.Microb Biotechnol. 2025 May;18(5):e70165. doi: 10.1111/1751-7915.70165. Microb Biotechnol. 2025. PMID: 40417767 Free PMC article.
-
Probiotic Limosilactobacillus reuteri KUB-AC5 decreases urothelial cell invasion and enhances macrophage killing of uropathogenic Escherichia coli in vitro study.Front Cell Infect Microbiol. 2024 Jul 18;14:1401462. doi: 10.3389/fcimb.2024.1401462. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39091675 Free PMC article.
-
Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications.Cells. 2025 May 16;14(10):731. doi: 10.3390/cells14100731. Cells. 2025. PMID: 40422234 Free PMC article. Review.
References
-
- Vallejo-Torres L., Pujol M., Shaw E., Wiegand I., Vigo J.M., Stoddart M., Grier S., Gibbs J., Vank C., Cuperus N., et al. Cost of hospitalised patients due to complicated urinary tract infections: A retrospective observational study in countries with high prevalence of multidrug-resistant Gram-negative bacteria: The combacte-magnet, rescuing study. BMJ Open. 2018;8:e020251. doi: 10.1136/bmjopen-2017-020251. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources