Celecoxib-Loaded Cubosomal Nanoparticles as a Therapeutic Approach for Staphylococcus aureus In Vivo Infection
- PMID: 37764091
- PMCID: PMC10535980
- DOI: 10.3390/microorganisms11092247
Celecoxib-Loaded Cubosomal Nanoparticles as a Therapeutic Approach for Staphylococcus aureus In Vivo Infection
Abstract
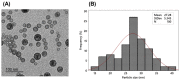
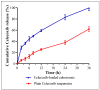
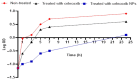
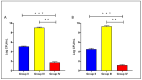
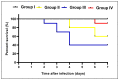
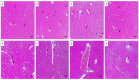
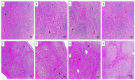
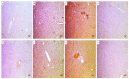
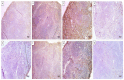
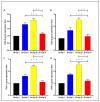
There is a great need for novel approaches to treating bacterial infections, due to the vast dissemination of resistance among pathogenic bacteria. Staphylococcus aureus are ubiquitous Gram-positive pathogenic bacteria and are rapidly acquiring antibiotic resistance. Here, celecoxib was encapsulated into cubosomal nanoparticles, and the particle morphology, size distribution, zeta potential, entrapment efficiency, and celecoxib release were evaluated in vitro. Also, a systemic infection model in mice elucidated the in vivo antibacterial action of the celecoxib cubosomes. Cubosomes are a nanotechnology-based delivery system which can adhere to the external peptidoglycan layers of Gram-positive bacteria and penetrate them. The size distribution investigation revealed that the prepared celecoxib-loaded cubosomes had a mean particle size of 128.15 ± 3.04 nm with a low polydispersity index of 0.235 ± 0.023. The zeta potential measurement showed that the prepared cubosomes had a negative surface charge of -17.50 ± 0.45, indicating a highly stable nanodispersion formation with little susceptibility to particle aggregation. The cubosomal dispersion exhibited an entrapment efficiency of 88.57 ± 2.36%. The transmission electron micrograph for the prepared celecoxib-loaded cubosomes showed a narrow size distribution for the cubosomal nanoparticles, which had a spherical shape and were non-aggregated. The tested cubosomes diminished the inflammation in the treated mice's liver and spleen tissues, as revealed by hematoxylin and eosin stain and Masson's trichrome stain. The immunostained tissues with nuclear factor kappa B and caspase-3 monoclonal antibodies revealed a marked decrease in these markers in the celecoxib-treated group, as it resulted in negative or weak immunostaining in liver and spleen that ranged from 4.54% to 17.43%. This indicates their inhibitory effect on the inflammatory pathway and apoptosis, respectively. Furthermore, they reduced the bacterial burden in the studied tissues. This is alongside a decrease in the inflammatory markers (interleukin-1 beta, interleukin-6, cyclooxygenase-2, and tumor necrosis factor-alpha) determined by ELISA and qRT-PCR. The IL-1β levels were 16.66 ± 0.5 pg/mg and 17 ± 0.9 pg/mg in liver and spleen, respectively. Also, IL-6 levels were 85 ± 3.2 pg/mg and 84 ± 2.4 pg/mg in liver and spleen, respectively. In conclusion, the current study introduced cubosomes as an approach for the formulation of celecoxib to enhance its in vivo antibacterial action by improving its oral bioavailability.
Keywords: bacterial infection; histological features; inflammatory markers; nanostructures; oral delivery system; repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Serwecińska L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water. 2020;12:3313. doi: 10.3390/w12123313. - DOI
-
- Attallah N.G., El-Kadem A.H., Negm W.A., Elekhnawy E., El-Masry T.A., Elmongy E.I., Altwaijry N., Alanazi A.S., Al-Hamoud G.A., Ragab A.E. Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study. Pharmaceuticals. 2021;14:1313. doi: 10.3390/ph14121313. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

